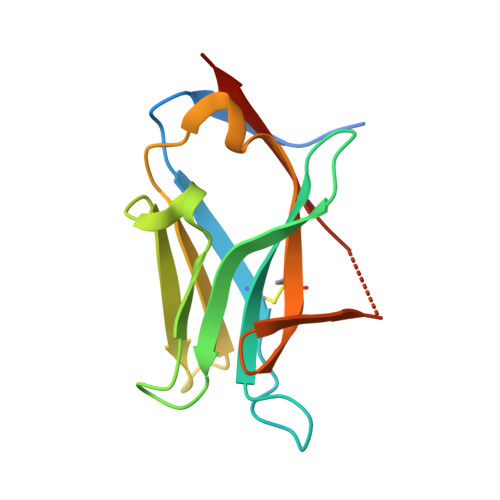
A selection and optimization strategy for single-domain antibodies targeting the PHF6 linear peptide within the tau intrinsically disordered protein.
Mortelecque, J., Zejneli, O., Begard, S., Simoes, M.C., ElHajjar, L., Nguyen, M., Cantrelle, F.X., Hanoulle, X., Rain, J.C., Colin, M., Gomes, C.M., Buee, L., Landrieu, I., Danis, C., Dupre, E.(2024) J Biological Chem 300: 107163-107163
- PubMed: 38484799
- DOI: https://doi.org/10.1016/j.jbc.2024.107163
- Primary Citation of Related Structures:
8OP0, 8OPI, 8PII - PubMed Abstract:
The use of variable domain of the heavy-chain of the heavy-chain-only antibodies (VHHs) as disease-modifying biomolecules in neurodegenerative disorders holds promises, including targeting of aggregation-sensitive proteins. Exploitation of their clinical values depends however on the capacity to deliver VHHs with optimal physico-chemical properties for their specific context of use. We described previously a VHH with high therapeutic potential in a family of neurodegenerative diseases called tauopathies. The activity of this promising parent VHH named Z70 relies on its binding within the central region of the tau protein. Accordingly, we carried out random mutagenesis followed by yeast two-hybrid screening to obtain optimized variants. The VHHs selected from this initial screen targeted the same epitope as VHH Z70 as shown using NMR spectroscopy and had indeed improved binding affinities according to dissociation constant values obtained by surface plasmon resonance spectroscopy. The improved affinities can be partially rationalized based on three-dimensional structures and NMR data of three complexes consisting of an optimized VHH and a peptide containing the tau epitope. Interestingly, the ability of the VHH variants to inhibit tau aggregation and seeding could not be predicted from their affinity alone. We indeed showed that the in vitro and in cellulo VHH stabilities are other limiting key factors to their efficacy. Our results demonstrate that only a complete pipeline of experiments, here described, permits a rational selection of optimized VHH variants, resulting in the selection of VHH variants with higher affinities and/or acting against tau seeding in cell models.
- CNRS EMR9002 - BSI - Integrative Structural Biology, Lille, France; Univ. Lille, Inserm, CHU Lille, Institut Pasteur de Lille, U1167 - RID-AGE - Risk Factors and Molecular Determinants of Aging-Related Diseases, Lille, France.
Organizational Affiliation: