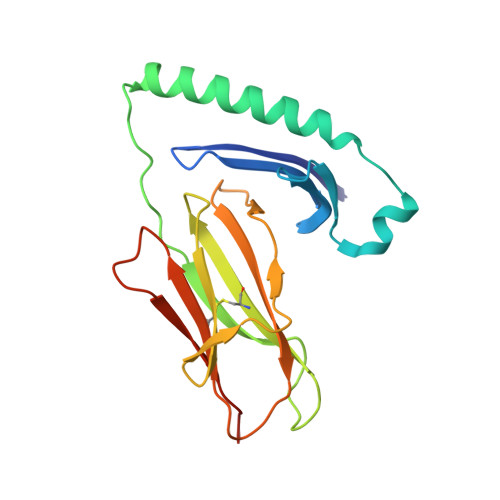
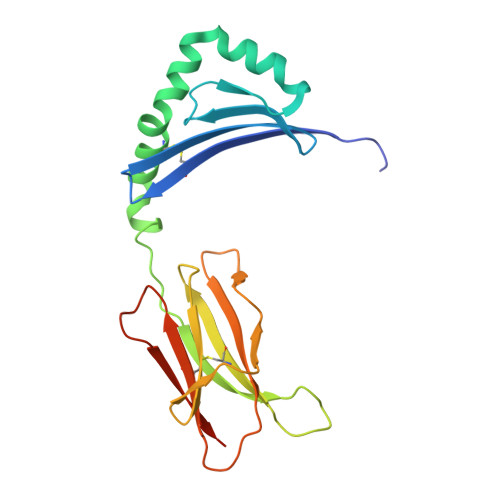
Molecular basis for increased susceptibility of Indigenous North Americans to seropositive rheumatoid arthritis.
Scally, S.W., Law, S.C., Ting, Y.T., Heemst, J.V., Sokolove, J., Deutsch, A.J., Bridie Clemens, E., Moustakas, A.K., Papadopoulos, G.K., Woude, D.V., Smolik, I., Hitchon, C.A., Robinson, D.B., Ferucci, E.D., Bernstein, C.N., Meng, X., Anaparti, V., Huizinga, T., Kedzierska, K., Reid, H.H., Raychaudhuri, S., Toes, R.E., Rossjohn, J., El-Gabalawy, H., Thomas, R.(2017) Ann Rheum Dis 76: 1915-1923
- PubMed: 28801345
- DOI: https://doi.org/10.1136/annrheumdis-2017-211300
- Primary Citation of Related Structures:
6ATF, 6ATI, 6ATZ - PubMed Abstract:
The pathogenetic mechanisms by which HLA-DRB1 alleles are associated with anticitrullinated peptide antibody (ACPA)-positive rheumatoid arthritis (RA) are incompletely understood. RA high-risk HLA-DRB1 alleles are known to share a common motif, the 'shared susceptibility epitope (SE)'. Here, the electropositive P4 pocket of HLA-DRB1 accommodates self-peptide residues containing citrulline but not arginine. HLA-DRB1 His/Phe13β stratifies with ACPA-positive RA, while His13βSer polymorphisms stratify with ACPA-negative RA and RA protection. Indigenous North American (INA) populations have high risk of early-onset ACPA-positive RA, whereby HLA-DRB1*04:04 and HLA-DRB1*14:02 are implicated as risk factors for RA in INA. However, HLA-DRB1*14:02 has a His13βSer polymorphism. Therefore, we aimed to verify this association and determine its molecular mechanism. HLA genotype was compared in 344 INA patients with RA and 352 controls. Structures of HLA-DRB1*1402-class II loaded with vimentin-64Arg 59-71 , vimentin-64Cit 59-71 and fibrinogen β-74Cit 69-81 were solved using X-ray crystallography. Vimentin-64Cit 59-71 -specific and vimentin 59-71 -specific CD4+ T cells were characterised by flow cytometry using peptide-histocompatibility leukocyte antigen (pHLA) tetramers. After sorting of antigen-specific T cells, TCRα and β-chains were analysed using multiplex, nested PCR and sequencing. ACPA + RA in INA was independently associated with HLA-DRB1*14:02 . Consequent to the His13βSer polymorphism and altered P4 pocket of HLA-DRB1*14:02, both citrulline and arginine were accommodated in opposite orientations. Oligoclonal autoreactive CD4+ effector T cells reactive with both citrulline and arginine forms of vimentin 59-71 were observed in patients with HLA-DRB1*14:02 + RA and at-risk ACPA - first-degree relatives. HLA-DRB1*14:02-vimentin 59-71 -specific and HLA-DRB1*14:02-vimentin-64Cit 59-71 -specific CD4+ memory T cells were phenotypically distinct populations. HLA-DRB1*14:02 broadens the capacity for citrullinated and native self-peptide presentation and T cell expansion, increasing risk of ACPA+ RA.
- Department of Biochemistry and Molecular Biology, Infection and Immunity Program, Biomedicine Discovery Institute Monash University, Clayton, Australia.
Organizational Affiliation: