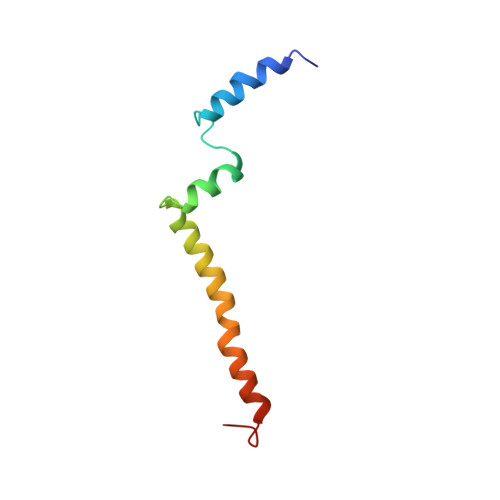
NMR Structure of the C-Terminal Transmembrane Domain of the HDL Receptor, SR-BI, and a Functionally Relevant Leucine Zipper Motif.
Chadwick, A.C., Jensen, D.R., Hanson, P.J., Lange, P.T., Proudfoot, S.C., Peterson, F.C., Volkman, B.F., Sahoo, D.(2017) Structure 25: 446-457
- PubMed: 28162952
- DOI: https://doi.org/10.1016/j.str.2017.01.001
- Primary Citation of Related Structures:
5KTF - PubMed Abstract:
The interaction of high-density lipoprotein (HDL) with its receptor, scavenger receptor BI (SR-BI), is critical for lowering plasma cholesterol levels and reducing the risk for cardiovascular disease. The HDL/SR-BI complex facilitates delivery of cholesterol into cells and is likely mediated by receptor dimerization. This work describes the use of nuclear magnetic resonance (NMR) spectroscopy to generate the first high-resolution structure of the C-terminal transmembrane domain of SR-BI. This region of SR-BI harbors a leucine zipper dimerization motif, which when mutated impairs the ability of the receptor to bind HDL and mediate cholesterol delivery. These losses in function correlate with the inability of SR-BI to form dimers. We also identify juxtamembrane regions of the extracellular domain of SR-BI that may interact with the lipid surface to facilitate cholesterol transport functions of the receptor.
- Department of Biochemistry, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, USA.
Organizational Affiliation: