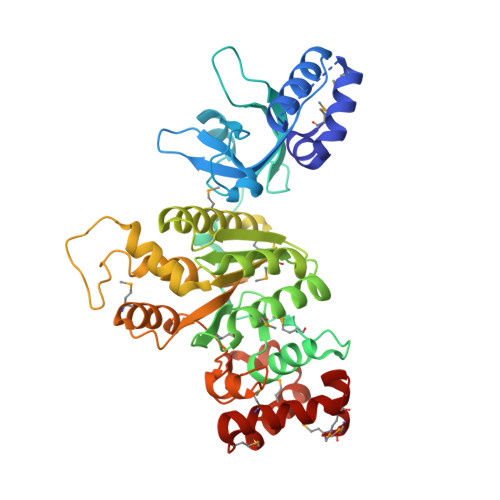
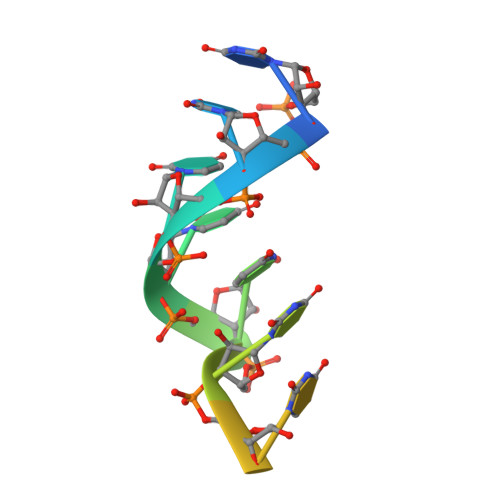
Molecular mechanisms of substrate-controlled ring dynamics and substepping in a nucleic acid-dependent hexameric motor.
Thomsen, N.D., Lawson, M.R., Witkowsky, L.B., Qu, S., Berger, J.M.(2016) Proc Natl Acad Sci U S A 113: E7691-E7700
- PubMed: 27856760
- DOI: https://doi.org/10.1073/pnas.1616745113
- Primary Citation of Related Structures:
5JJI, 5JJK, 5JJL - PubMed Abstract:
Ring-shaped hexameric helicases and translocases support essential DNA-, RNA-, and protein-dependent transactions in all cells and many viruses. How such systems coordinate ATPase activity between multiple subunits to power conformational changes that drive the engagement and movement of client substrates is a fundamental question. Using the Escherichia coli Rho transcription termination factor as a model system, we have used solution and crystallographic structural methods to delineate the range of conformational changes that accompany distinct substrate and nucleotide cofactor binding events. Small-angle X-ray scattering data show that Rho preferentially adopts an open-ring state in solution and that RNA and ATP are both required to cooperatively promote ring closure. Multiple closed-ring structures with different RNA substrates and nucleotide occupancies capture distinct catalytic intermediates accessed during translocation. Our data reveal how RNA-induced ring closure templates a sequential ATP-hydrolysis mechanism, provide a molecular rationale for how the Rho ATPase domains distinguishes between distinct RNA sequences, and establish structural snapshots of substepping events in a hexameric helicase/translocase.
- Department of Molecular and Cell Biology, University of California, Berkeley, CA 94720.
Organizational Affiliation: