Fragment-Based Lead Discovery Using X-Ray Crystallography
Hartshorn, M.J., Murray, C.W., Cleasby, A., Frederickson, M., Tickle, I.J., Jhoti, H.(2005) J Med Chem 48: 403
- PubMed: 15658854
- DOI: https://doi.org/10.1021/jm0495778
- Primary Citation of Related Structures:
1W7H, 1WAX, 1WAY, 1WBG, 1WBO, 1WBU, 1WCC - PubMed Abstract:
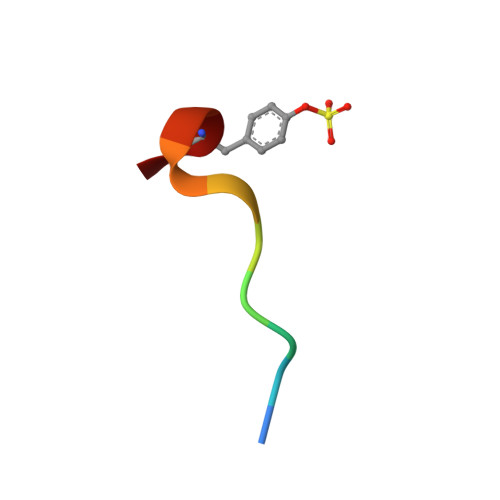
Fragment screening offers an alternative to traditional screening for discovering new leads in drug discovery programs. This paper describes a fragment screening methodology based on high throughput X-ray crystallography. The method is illustrated against five proteins (p38 MAP kinase, CDK2, thrombin, ribonuclease A, and PTP1B). The fragments identified have weak potency (>100 microM) but are efficient binders relative to their size and may therefore represent suitable starting points for evolution to good quality lead compounds. The examples illustrate that a range of molecular interactions (i.e., lipophilic, charge-charge, neutral hydrogen bonds) can drive fragment binding and also that fragments can induce protein movement. We believe that the method has great potential for the discovery of novel lead compounds against a range of targets, and the companion paper illustrates how lead compounds have been identified for p38 MAP kinase starting from fragments such as those described in this paper.
- Astex Technology, 436 Cambridge Science Park, Milton Road, Cambridge, CB4 0QA, United Kingdom.
Organizational Affiliation: