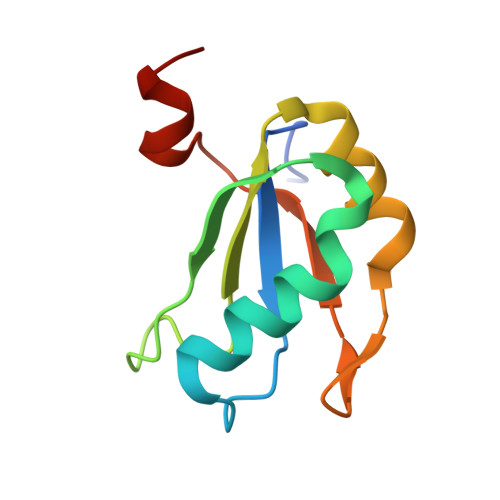
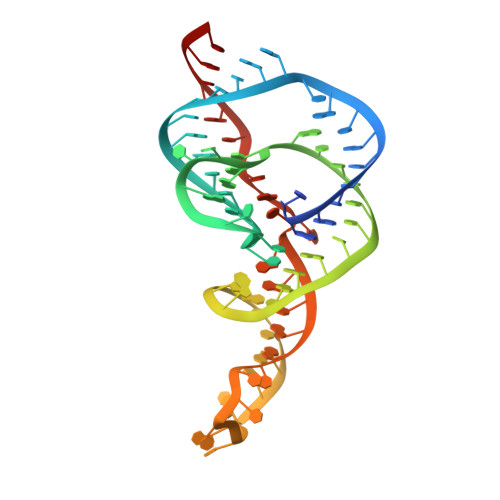
A Conformational Switch controls hepatitis delta virus ribozyme catalysis
Ke, A., Zhou, K., Ding, F., Cate, J.H., Doudna, J.A.(2004) Nature 429: 201-205
- PubMed: 15141216
- DOI: https://doi.org/10.1038/nature02522
- Primary Citation of Related Structures:
1SJ3, 1SJ4, 1SJF, 1VBX, 1VBY, 1VBZ, 1VC0, 1VC5, 1VC6 - PubMed Abstract:
Ribozymes enhance chemical reaction rates using many of the same catalytic strategies as protein enzymes. In the hepatitis delta virus (HDV) ribozyme, site-specific self-cleavage of the viral RNA phosphodiester backbone requires both divalent cations and a cytidine nucleotide. General acid-base catalysis, substrate destabilization and global and local conformational changes have all been proposed to contribute to the ribozyme catalytic mechanism. Here we report ten crystal structures of the HDV ribozyme in its pre-cleaved state, showing that cytidine is positioned to activate the 2'-OH nucleophile in the precursor structure. This observation supports its proposed role as a general base in the reaction mechanism. Comparison of crystal structures of the ribozyme in the pre- and post-cleavage states reveals a significant conformational change in the RNA after cleavage and that a catalytically critical divalent metal ion from the active site is ejected. The HDV ribozyme has remarkable chemical similarity to protein ribonucleases and to zymogens for which conformational dynamics are integral to biological activity. This finding implies that RNA structural rearrangements control the reactivity of ribozymes and ribonucleoprotein enzymes.
- Department of Molecular and Cell Biology, University of California at Berkeley, Berkeley, California 94705, USA.
Organizational Affiliation: