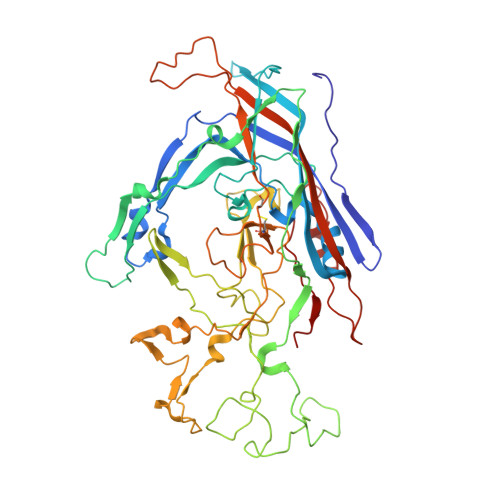
Structures of host range-controlling regions of the capsids of canine and feline parvoviruses and mutants.
Govindasamy, L., Hueffer, K., Parrish, C.R., Agbandje-McKenna, M.(2003) J Virol 77: 12211-12221
- PubMed: 14581558
- DOI: https://doi.org/10.1128/jvi.77.22.12211-12221.2003
- Primary Citation of Related Structures:
1P5W, 1P5Y - PubMed Abstract:
Canine parvovirus (CPV) and feline panleukopenia virus (FPV) differ in their ability to infect dogs and dog cells. Canine cell infection is a specific property of CPV and depends on the ability of the virus to bind the canine transferrin receptor (TfR), as well as other unidentified factors. Three regions in the capsid structure, located around VP2 residues 93, 300, and 323, can all influence canine TfR binding and canine cell infection. These regions were compared in the CPV and FPV capsid structures that have been determined, as well as in two new structures of CPV capsids that contain substitutions of the VP2 Asn-93 to Asp and Arg, respectively. The new structures, determined by X-ray crystallography to 3.2 and 3.3 A resolutions, respectively, clearly showed differences in the interactions of residue 93 with an adjacent loop on the capsid surface. Each of the three regions show small differences in structure, but each appears to be structurally independent of the others, and the changes likely act together to affect the ability of the capsid to bind the canine TfR and to infect canine cells. This emphasizes the complex nature of capsid alterations that change the virus-cell interaction to allow infection of cells from different hosts.
- Department of Biochemistry and Molecular Biology, Center for Structural Biology, College of Medicine, University of Florida, Gainesville, Florida 32610, USA.
Organizational Affiliation: