Structural and functional analyses of Rubisco from arctic diatom species reveal unusual posttranslational modifications.
Valegard, K., Andralojc, P.J., Haslam, R.P., Pearce, F.G., Eriksen, G.K., Madgwick, P.J., Kristoffersen, A.K., van Lun, M., Klein, U., Eilertsen, H.C., Parry, M.A.J., Andersson, I.(2018) J Biol Chem 293: 13033-13043
- PubMed: 29925588
- DOI: https://doi.org/10.1074/jbc.RA118.003518
- Primary Citation of Related Structures:
5MZ2, 5N9Z, 5OYA, 6FTL - PubMed Abstract:
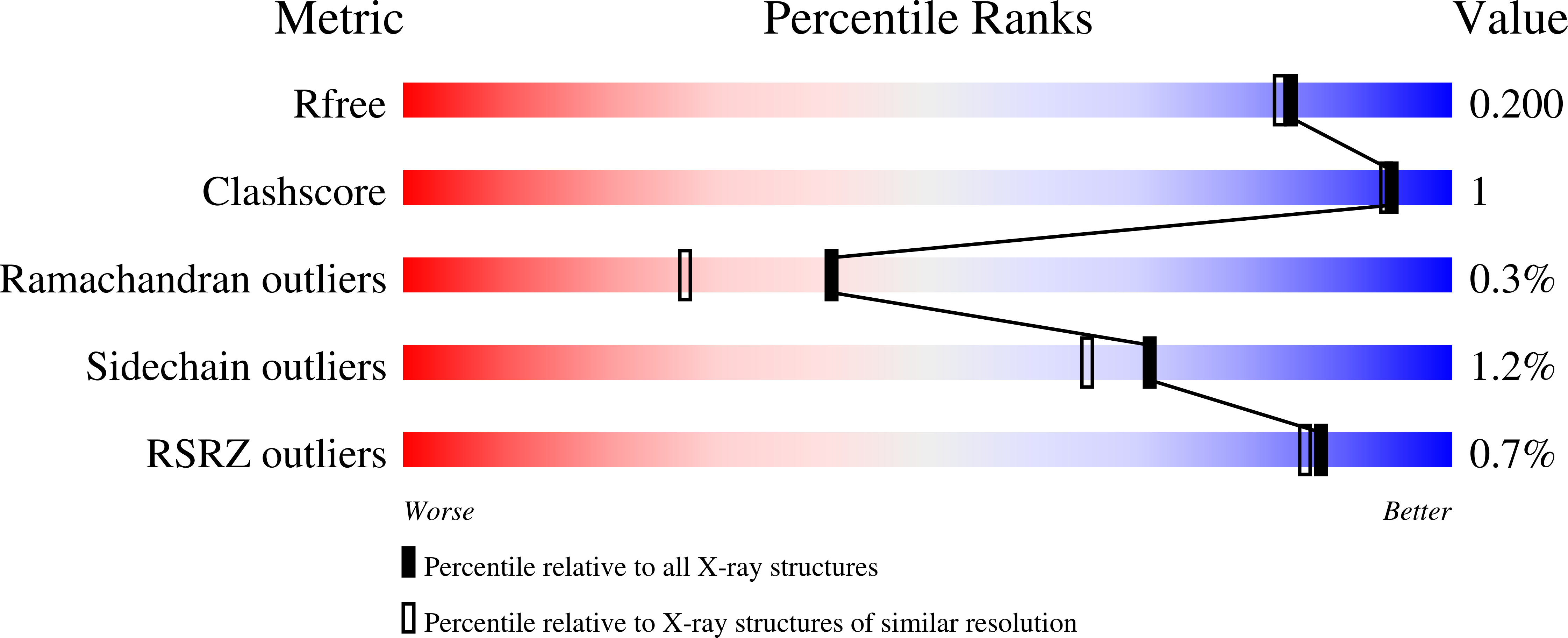
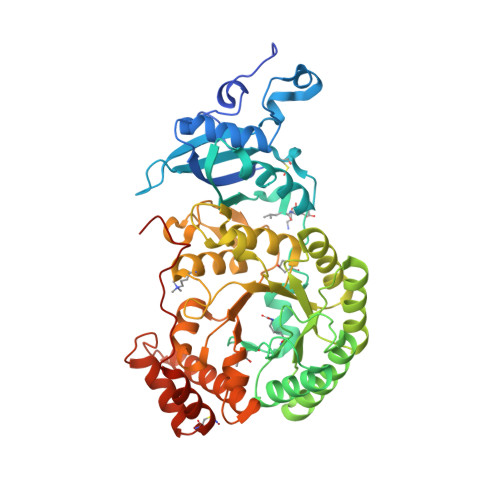
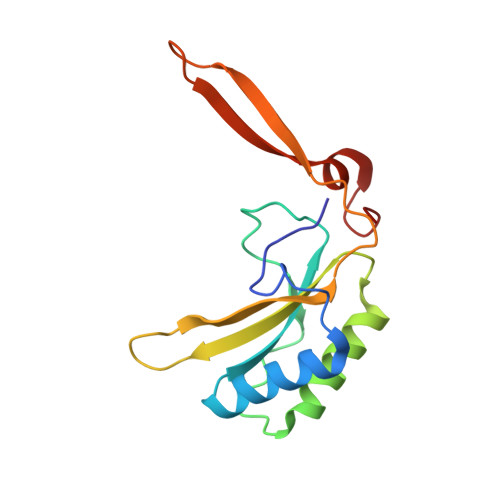
The catalytic performance of the major CO 2 -assimilating enzyme, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), restricts photosynthetic productivity. Natural diversity in the catalytic properties of Rubisco indicates possibilities for improvement. Oceanic phytoplankton contain some of the most efficient Rubisco enzymes, and diatoms in particular are responsible for a significant proportion of total marine primary production as well as being a major source of CO 2 sequestration in polar cold waters. Until now, the biochemical properties and three-dimensional structures of Rubisco from diatoms were unknown. Here, diatoms from arctic waters were collected, cultivated, and analyzed for their CO 2 -fixing capability. We characterized the kinetic properties of five and determined the crystal structures of four Rubiscos selected for their high CO 2 -fixing efficiency. The DNA sequences of the rbc L and rbc S genes of the selected diatoms were similar, reflecting their close phylogenetic relationship. The V max and K m for the oxygenase and carboxylase activities at 25 °C and the specificity factors ( S c/o ) at 15, 25, and 35 °C were determined. The S c/o values were high, approaching those of mono- and dicot plants, thus exhibiting good selectivity for CO 2 relative to O 2 Structurally, diatom Rubiscos belong to form I C/D, containing small subunits characterized by a short βA-βB loop and a C-terminal extension that forms a β-hairpin structure (βE-βF loop). Of note, the diatom Rubiscos featured a number of posttranslational modifications of the large subunit, including 4-hydroxyproline, β-hydroxyleucine, hydroxylated and nitrosylated cysteine, mono- and dihydroxylated lysine, and trimethylated lysine. Our studies suggest adaptation toward achieving efficient CO 2 fixation in arctic diatom Rubiscos.
Organizational Affiliation:
From the Department of Cell and Molecular Biology, Uppsala University, Box 596, S-751 24 Uppsala, Sweden.