Environmental contributions to proton sharing in protein low barrier hydrogen bonds
Lin, J., Gerlits, O., Kneller, D.W., Weiss, K.L., Coates, L., Hix, M.A., Effah, S.Y., Kovalevsky, A., Walker, A.R., Wilson, M.A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein/nucleic acid deglycase 3 | 199 | Escherichia coli | Mutation(s): 1 Gene Names: yajL, thiJ, b0424, JW5057 EC: 3.1.2 (PDB Primary Data), 3.5.1 (PDB Primary Data), 3.5.1.124 (PDB Primary Data) | 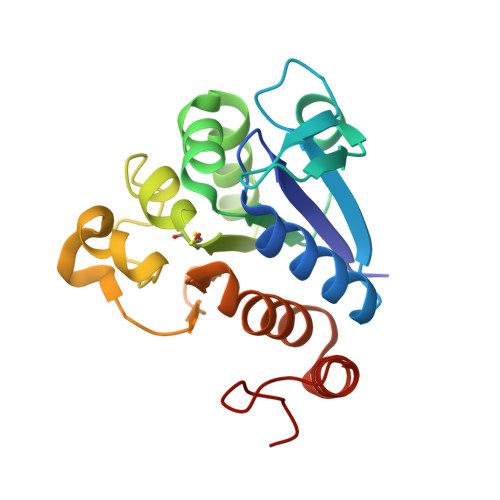 | |
UniProt | |||||
Find proteins for Q46948 (Escherichia coli (strain K12)) Explore Q46948 Go to UniProtKB: Q46948 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q46948 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| EDO Query on EDO | C [auth A] D [auth A] E [auth A] L [auth B] M [auth B] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| CL Query on CL | F [auth A], G [auth A], H [auth A], I [auth A], O [auth B] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| MG Query on MG | J [auth A], K [auth A], P [auth B] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CSO Query on CSO | A, B | L-PEPTIDE LINKING | C3 H7 N O3 S |  | CYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 43.723 | α = 90 |
| b = 78.335 | β = 90 |
| c = 99.614 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | R35GM153337 |