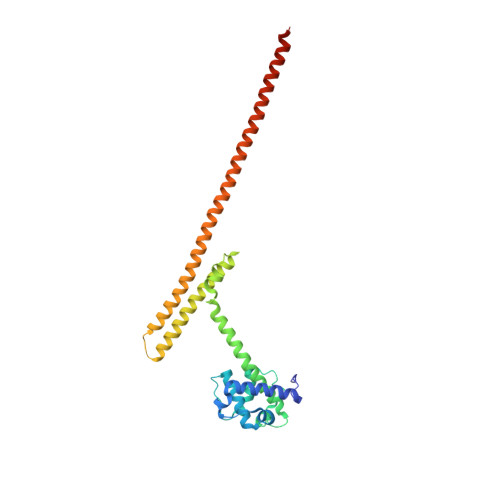
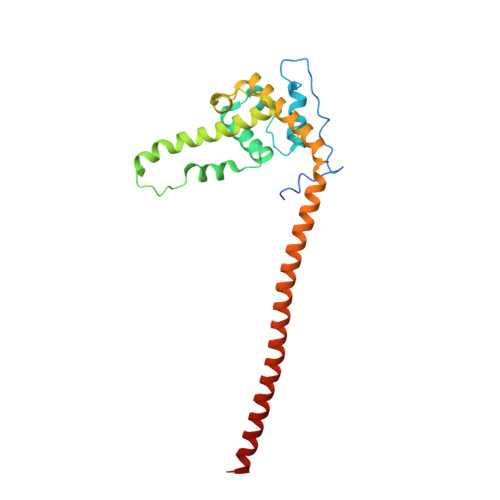
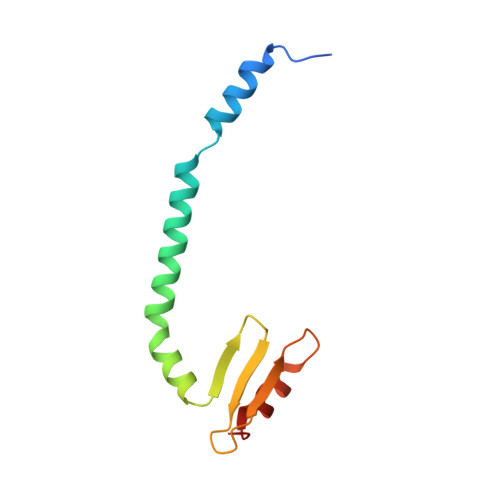
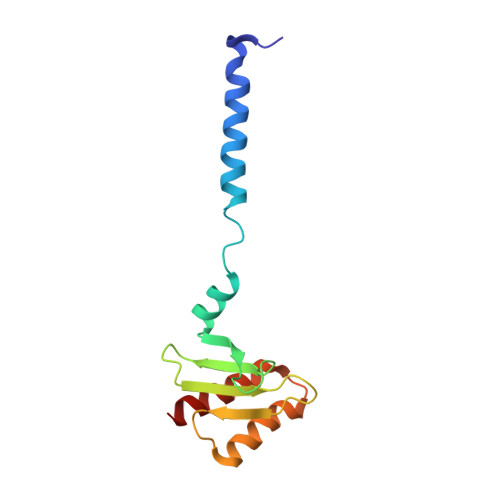
A communication hub for phosphoregulation of kinetochore-microtubule attachment.
Zahm, J.A., Harrison, S.C.(2024) Curr Biol 34: 2308-2318.e6
- PubMed: 38776904
- DOI: https://doi.org/10.1016/j.cub.2024.04.067
- Primary Citation of Related Structures:
8V10, 8V11 - PubMed Abstract:
The Mps1 and Aurora B kinases regulate and monitor kinetochore attachment to spindle microtubules during cell division, ultimately ensuring accurate chromosome segregation. In yeast, the critical spindle attachment components are the Ndc80 and Dam1 complexes (Ndc80c and DASH/Dam1c, respectively). Ndc80c is a 600-Å-long heterotetramer that binds microtubules through a globular "head" at one end and centromere-proximal kinetochore components through a globular knob at the other end. Dam1c is a heterodecamer that forms a ring of 16-17 protomers around the shaft of the single kinetochore microtubule in point-centromere yeast. The ring coordinates the approximately eight Ndc80c rods per kinetochore. In published work, we showed that a site on the globular "head" of Ndc80c, including residues from both Ndc80 and Nuf2, binds a bipartite segment in the long C-terminal extension of Dam1. Results reported here show, both by in vitro binding experiments and by crystal structure determination, that the same site binds a conserved segment in the long N-terminal extension of Mps1. It also binds, less tightly, a conserved segment in the N-terminal extension of Ipl1 (yeast Aurora B). Together with results from experiments in yeast cells and from biochemical assays reported in two accompanying papers, the structures and graded affinities identify a communication hub for ensuring uniform bipolar attachment and for signaling anaphase onset.
- Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA.
Organizational Affiliation: