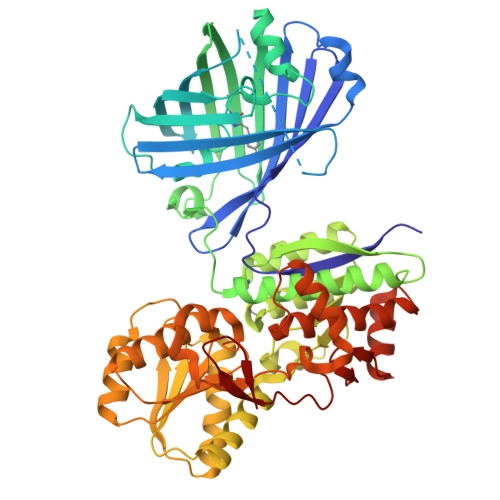
NeIle, a Genetically Encoded Indicator for Branched-Chain Amino Acids Based on mNeonGreen Fluorescent Protein and LIVBP Protein.
Asanova, A.N., Subach, O.M., Myachina, S.A., Evteeva, M.A., Gunitseva, N.M., Borisova, A.A., Patrushev, M.V., Vlaskina, A.V., Nikolaeva, A.Y., Yang, L., Gabdulkhakov, A., Dronova, E., Samygina, V.R., Xiao, X., Zhao, H., Piatkevich, K.D., Subach, F.V.(2024) ACS Sens 9: 5135-5147
- PubMed: 39400357
- DOI: https://doi.org/10.1021/acssensors.4c01055
- Primary Citation of Related Structures:
8Z0G, 9JTI - PubMed Abstract:
Branched-chain amino acids (BCAAs) play an important role in the functioning of mammalian cells and the central nervous system. However, available genetically encoded indicators for BCAAs are based on Förster resonance energy transfer and have a limited dynamic range. We developed a single fluorescent protein-based sensor for BCAAs, called NeIle, which is composed of circularly permutated mNeonGreen protein inserted into the leucine-isoleucine-valine binding protein (LIVBP) from Escherichia coli bacteria. In solution, the NeIle indicator displayed a positive fluorescence response to adding isoleucine, leucine, and valin amino acids with high Δ F / F dynamic ranges of 27-, 19-, and 11-fold and the corresponding affinity values of 5.0, 2.9, and 75 mM, respectively. The spectral and biochemical properties of the NeIle indicator were characterized in solution. We characterized the brightness of the NeIle indicator in living mammalian cells, including cultured neurons. Using the NeIle indicator, we successfully visualized the dynamics of isoleucine transients in different organelles of mammalian cells. We obtained and analyzed the X-ray crystal structure of the NeIle indicator in an isoleucine-bound state. Structure-guided directed mutagenesis of the NeIle indicator revealed the basis of its fluorescence response and selectivity to isoleucine.
- Complex of NBICS Technologies, National Research Center "Kurchatov Institute", Moscow 123182, Russia.
Organizational Affiliation: