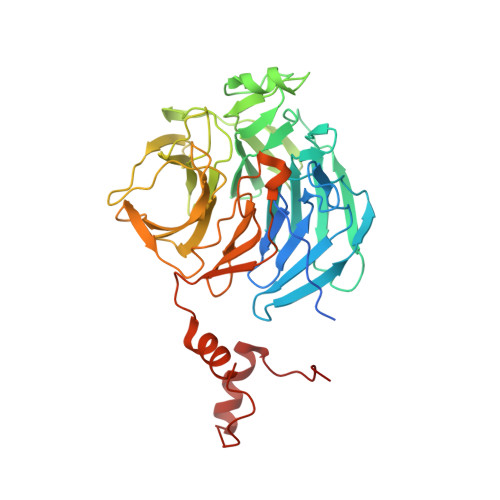
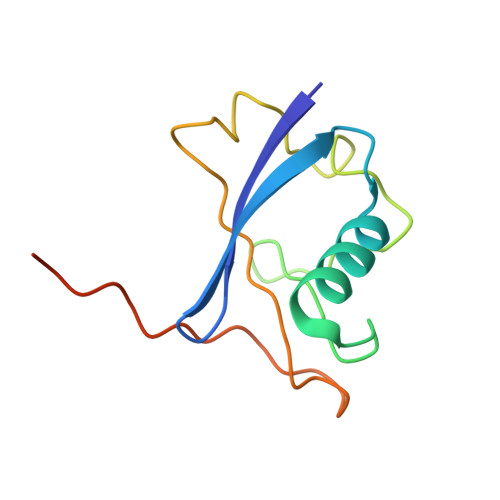
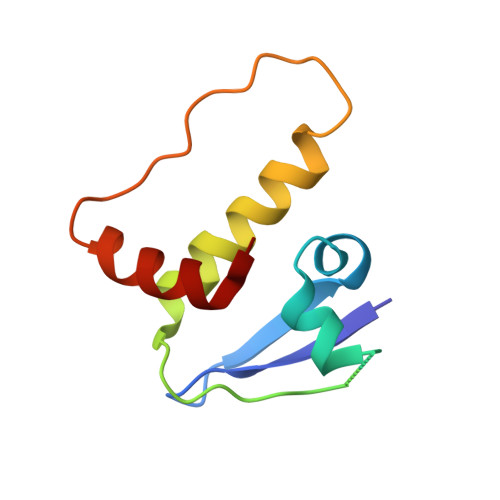
Co-opting the E3 ligase KLHDC2 for targeted protein degradation by small molecules.
Hickey, C.M., Digianantonio, K.M., Zimmermann, K., Harbin, A., Quinn, C., Patel, A., Gareiss, P., Chapman, A., Tiberi, B., Dobrodziej, J., Corradi, J., Cacace, A.M., Langley, D.R., Bekes, M.(2024) Nat Struct Mol Biol 31: 311-322
- PubMed: 38177675
- DOI: https://doi.org/10.1038/s41594-023-01146-w
- Primary Citation of Related Structures:
8SGE, 8SGF, 8SH2 - PubMed Abstract:
Targeted protein degradation (TPD) by PROTAC (proteolysis-targeting chimera) and molecular glue small molecules is an emerging therapeutic strategy. To expand the roster of E3 ligases that can be utilized for TPD, we describe the discovery and biochemical characterization of small-molecule ligands targeting the E3 ligase KLHDC2. Furthermore, we functionalize these KLHDC2-targeting ligands into KLHDC2-based BET-family and AR PROTAC degraders and demonstrate KLHDC2-dependent target-protein degradation. Additionally, we offer insight into the assembly of the KLHDC2 E3 ligase complex. Using biochemical binding studies, X-ray crystallography and cryo-EM, we show that the KLHDC2 E3 ligase assembles into a dynamic tetramer held together via its own C terminus, and that this assembly can be modulated by substrate and ligand engagement.
- Arvinas, Inc, New Haven, CT, USA.
Organizational Affiliation: