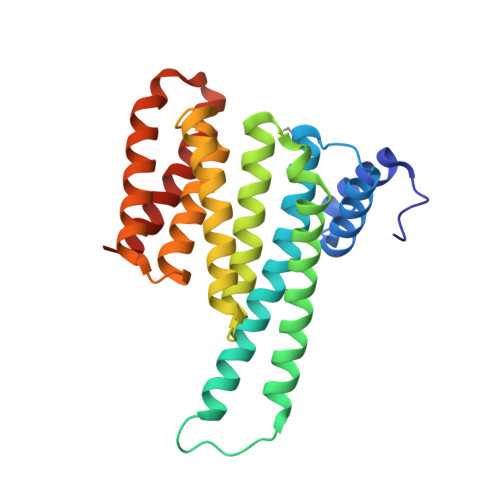
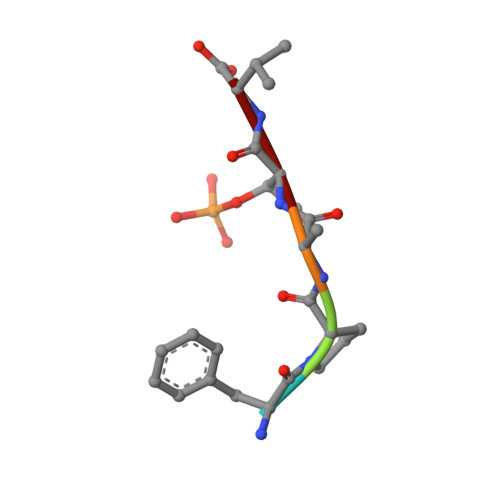
From Tethered to Freestanding Stabilizers of 14-3-3 Protein-Protein Interactions through Fragment Linking.
Visser, E.J., Jaishankar, P., Sijbesma, E., Pennings, M.A.M., Vandenboorn, E.M.F., Guillory, X., Neitz, R.J., Morrow, J., Dutta, S., Renslo, A.R., Brunsveld, L., Arkin, M.R., Ottmann, C.(2023) Angew Chem Int Ed Engl 62: e202308004-e202308004
- PubMed: 37455289
- DOI: https://doi.org/10.1002/anie.202308004
- Primary Citation of Related Structures:
8BWJ, 8BWX, 8BWZ, 8BX0, 8BX3, 8BX4, 8BXI, 8BXM, 8BXN, 8BXO, 8BXQ, 8BXS, 8BY9, 8BYB, 8BYC, 8BYD, 8BYE, 8BYF, 8BYG, 8BYO, 8BYY, 8BYZ, 8BZ0, 8BZ9, 8BZA, 8BZB, 8BZW, 8C04, 8C0K, 8C4F, 8C4G - PubMed Abstract:
Small-molecule stabilization of protein-protein interactions (PPIs) is a promising strategy in chemical biology and drug discovery. However, the systematic discovery of PPI stabilizers remains a largely unmet challenge. Herein we report a fragment-linking approach targeting the interface of 14-3-3 and a peptide derived from the estrogen receptor alpha (ERα) protein. Two classes of fragments-a covalent and a noncovalent fragment-were co-crystallized and subsequently linked, resulting in a noncovalent hybrid molecule in which the original fragment interactions were largely conserved. Supported by 20 crystal structures, this initial hybrid molecule was further optimized, resulting in selective, 25-fold stabilization of the 14-3-3/ERα interaction. The high-resolution structures of both the single fragments, their co-crystal structures and those of the linked fragments document a feasible strategy to develop orthosteric PPI stabilizers by linking to an initial tethered fragment.
- Laboratory of Chemical Biology, Department of Biomedical Engineering and Institute for Complex Molecular Systems (ICMS), Eindhoven University of Technology, Den Dolech 2, 5612, AZ Eindhoven, The Netherlands.
Organizational Affiliation: