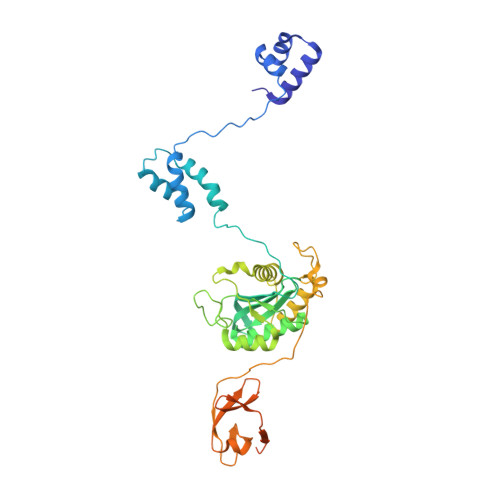
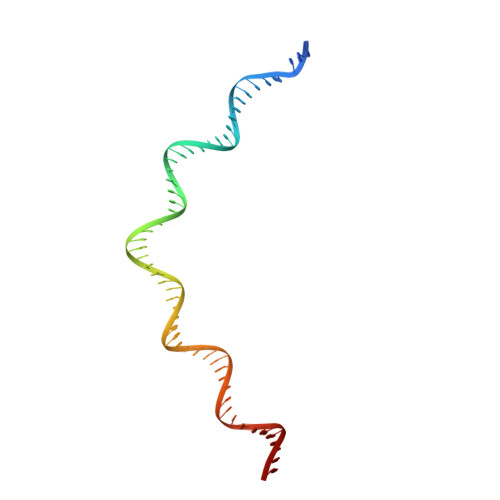
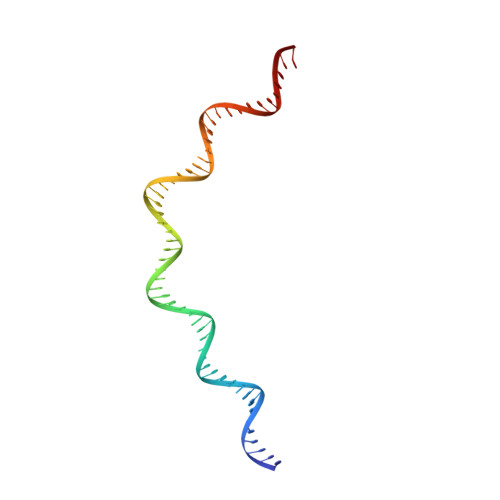
IS21 family transposase cleaved donor complex traps two right-handed superhelical crossings.
Spinola-Amilibia, M., Araujo-Bazan, L., de la Gandara, A., Berger, J.M., Arias-Palomo, E.(2023) Nat Commun 14: 2335-2335
- PubMed: 37087515
- DOI: https://doi.org/10.1038/s41467-023-38071-x
- Primary Citation of Related Structures:
8B4H - PubMed Abstract:
Transposases are ubiquitous enzymes that catalyze DNA rearrangement events with broad impacts on gene expression, genome evolution, and the spread of drug-resistance in bacteria. Here, we use biochemical and structural approaches to define the molecular determinants by which IstA, a transposase present in the widespread IS21 family of mobile elements, catalyzes efficient DNA transposition. Solution studies show that IstA engages the transposon terminal sequences to form a high-molecular weight complex and promote DNA integration. A 3.4 Å resolution structure of the transposase bound to transposon ends corroborates our biochemical findings and reveals that IstA self-assembles into a highly intertwined tetramer that synapses two supercoiled terminal inverted repeats. The three-dimensional organization of the IstA•DNA cleaved donor complex reveals remarkable similarities with retroviral integrases and classic transposase systems, such as Tn7 and bacteriophage Mu, and provides insights into IS21 transposition.
- Department of Structural & Chemical Biology, Centro de Investigaciones Biológicas Margarita Salas, CSIC, Madrid, 28040, Spain.
Organizational Affiliation: