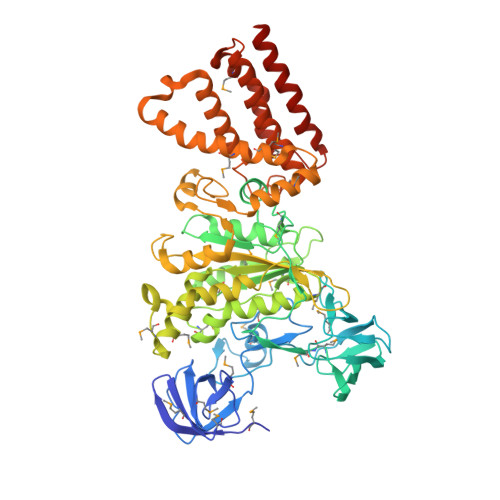
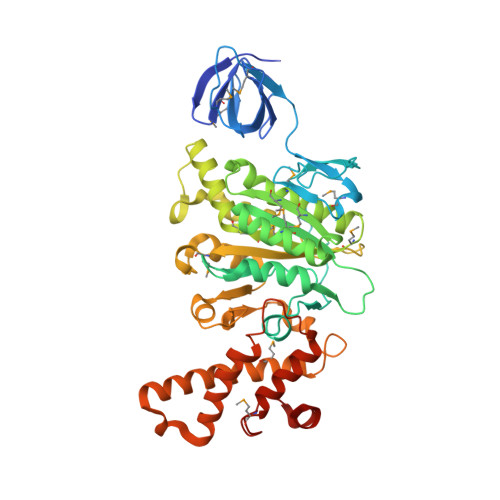
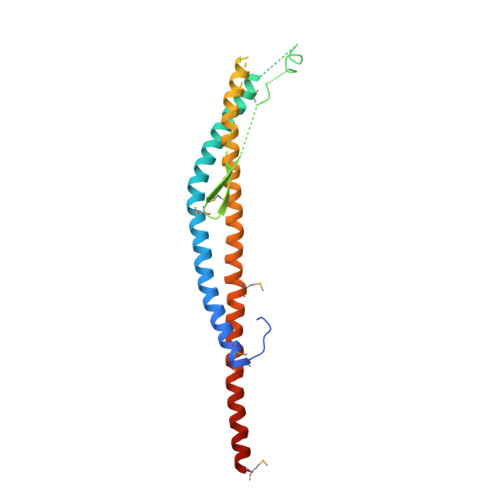
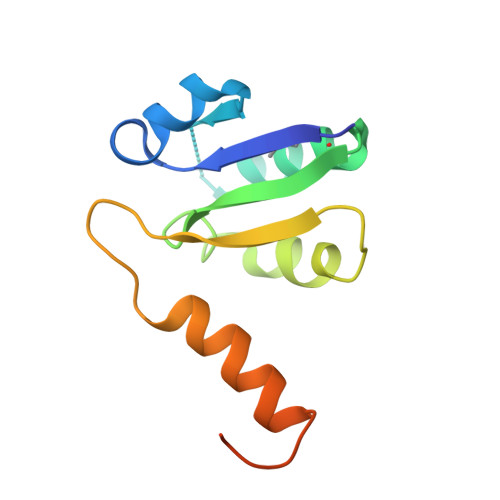
Revealing a Hidden Intermediate of Rotatory Catalysis with X-ray Crystallography and Molecular Simulations.
Shekhar, M., Gupta, C., Suzuki, K., Chan, C.K., Murata, T., Singharoy, A.(2022) ACS Cent Sci 8: 915-925
- PubMed: 35912346
- DOI: https://doi.org/10.1021/acscentsci.1c01599
- Primary Citation of Related Structures:
7VW7 - PubMed Abstract:
The mechanism of rotatory catalysis in ATP-hydrolyzing molecular motors remains an unresolved puzzle in biological energy transfer. Notwithstanding the wealth of available biochemical and structural information inferred from years of experiments, knowledge on how the coupling between the chemical and mechanical steps within motors enforces directional rotatory movements remains fragmentary. Even more contentious is to pinpoint the rate-limiting step of a multistep rotation process. Here, using vacuolar or V 1 -type hexameric ATPase as an exemplary rotational motor, we present a model of the complete 4-step conformational cycle involved in rotatory catalysis. First, using X-ray crystallography, a new intermediate or "dwell" is identified, which enables the release of an inorganic phosphate (or P i ) after ATP hydrolysis. Using molecular dynamics simulations, this new dwell is placed in a sequence with three other crystal structures to derive a putative cyclic rotation path. Free-energy simulations are employed to estimate the rate of the hexameric protein transformations and delineate allosteric effects that allow new reactant ATP entry only after hydrolysis product exit. An analysis of transfer entropy brings to light how the side-chain-level interactions transcend into larger-scale reorganizations, highlighting the role of the ubiquitous arginine-finger residues in coupling chemical and mechanical information. An inspection of all known rates encompassing the 4-step rotation mechanism implicates the overcoming of the ADP interactions with V 1 -ATPase to be the rate-limiting step of motor action.
- Center for Development of Therapeutics, Broad Institute of MIT and Harvard, 415 Main Street, Cambridge, Massachusetts 02142, United States.
Organizational Affiliation: