Biochemical and Structural Characterization of Thermostable GH159 Glycoside Hydrolases Exhibiting alpha-L-Arabinofuranosidase Activity.
Baudrexl, M., Fida, T., Berk, B., Schwarz, W.H., Zverlov, V.V., Groll, M., Liebl, W.(2022) Front Mol Biosci 9: 907439-907439
- PubMed: 35847984
- DOI: https://doi.org/10.3389/fmolb.2022.907439
- Primary Citation of Related Structures:
7ZEI - PubMed Abstract:
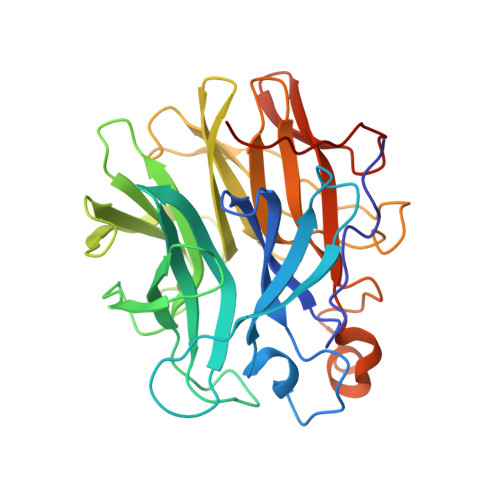
Functional, biochemical, and preliminary structural properties are reported for three glycoside hydrolases of the recently described glycoside hydrolase (GH) family 159. The genes were cloned from the genomic sequences of different Caldicellulosiruptor strains. This study extends the spectrum of functions of GH159 enzymes. The only activity previously reported for GH159 was hydrolytic activity on β-galactofuranosides. Activity screening using a set of para -nitrophenyl ( p NP) glycosides suggested additional arabinosidase activity on substrates with arabinosyl residues, which has not been previously reported for members of GH159. Even though the thermophilic enzymes investigated- Cs_ Gaf159A, Ch_ Gaf159A, and Ck _Gaf159A-cleaved p NP-α-l-arabinofuranoside, they were only weakly active on arabinogalactan, and they did not cleave arabinose from arabinan, arabinoxylan, or gum arabic. However, the enzymes were able to hydrolyze the α-1,3-linkage in different arabinoxylan-derived oligosaccharides (AXOS) with arabinosylated xylose at the non-reducing end (A 3 X, A 2,3 XX), suggesting their role in the intracellular hydrolysis of oligosaccharides. Crystallization and structural analysis of the apo form of one of the Caldicellulosiruptor enzymes, Ch _Gaf159A, enabled the elucidation of the first 3D structure of a GH159 member. This work revealed a five-bladed β-propeller structure for GH159 enzymes. The 3D structure and its substrate-binding pocket also provides an explanation at the molecular level for the observed exo-activity of the enzyme. Furthermore, the structural data enabled the prediction of the catalytic amino acids. This was supported by the complete inactivation by mutation of residues D19, D142, and E190 of Ch _Gaf159A.
- Chair of Microbiology, Technical University of Munich, Freising, Germany.
Organizational Affiliation: