Interaction between DLC-1 and SAO-1 facilitates CED-4 translocation during apoptosis in the Caenorhabditis elegans germline.
Zhang, D., Yang, H., Jiang, L., Zhao, C., Wang, M., Hu, B., Yu, C., Wei, Z., Tse, Y.C.(2022) Cell Death Discov 8: 441-441
- PubMed: 36323675
- DOI: https://doi.org/10.1038/s41420-022-01233-9
- Primary Citation of Related Structures:
7Y8W - PubMed Abstract:
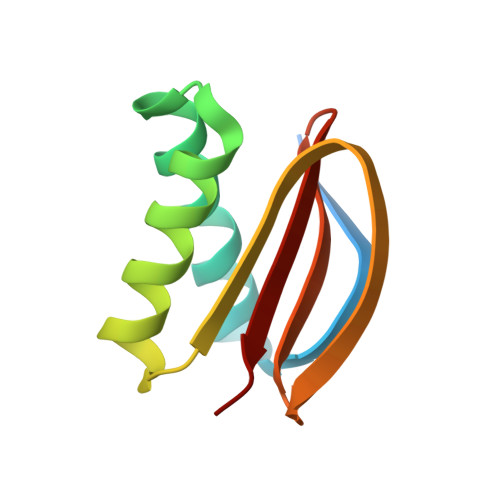
Apoptosis is one of the major forms of programmed cell death, and it serves vital biological functions in multicellular animal and plant cells. The core mechanism of apoptosis is highly conserved in metazoans, where the translocation of CED-4/Apaf-1 from mitochondria to the nuclear membrane is required to initiate and execute apoptosis. However, the underlying molecular mechanisms of this translocation are poorly understood. In this study, we showed that SAO-1 binds DLC-1 and prevents its degradation to promote apoptosis in C. elegans germ cells. We demonstrated that SAO-1 and DLC-1 regulate CED-4/Apaf-1 nuclear membrane accumulation during apoptosis. Isothermal titration calorimetry-based assay and high-resolution crystal structure analysis further revealed that SAO-1 interacted with DLC-1 to form a 2:4 complex: each of the two β-sheets in the SAO-1 peptide interacted with two DLC-1 dimers. Point mutations at the SAO-1-DLC-1 binding interface significantly inhibited apoptotic corpse formation and CED-4 nuclear membrane accumulation within C. elegans germ cells. In conclusion, our study provides a new perspective on the regulation of CED-4-mediated apoptosis.
- School of Life Science and Technology, Harbin Institute of Technology, Harbin, 150001, China.
Organizational Affiliation: