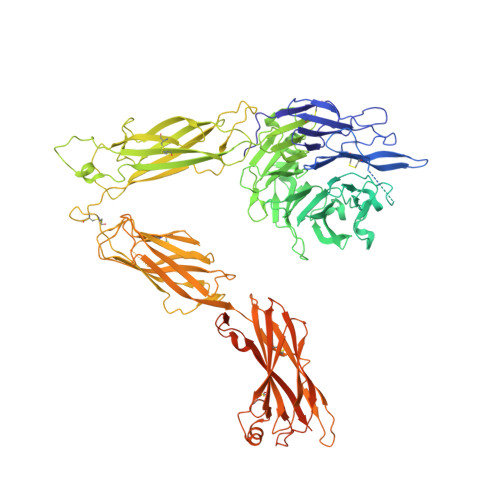
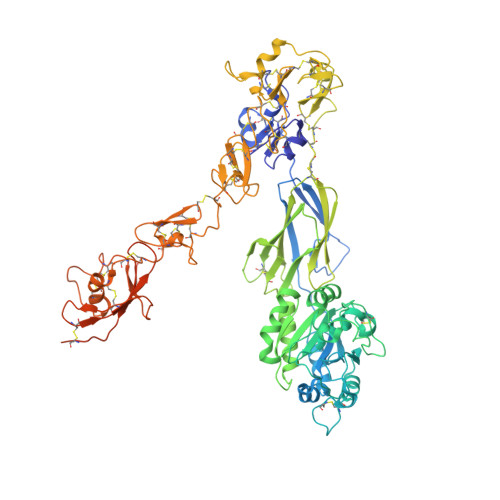
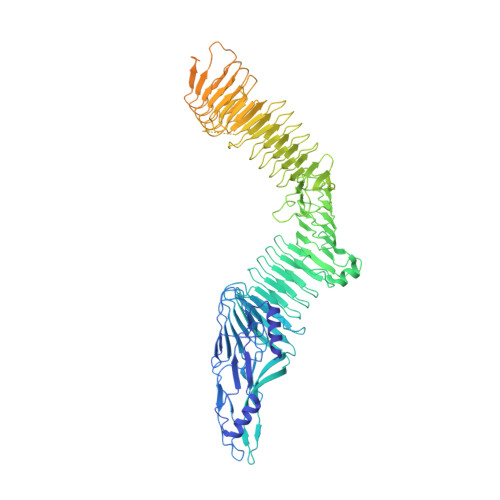
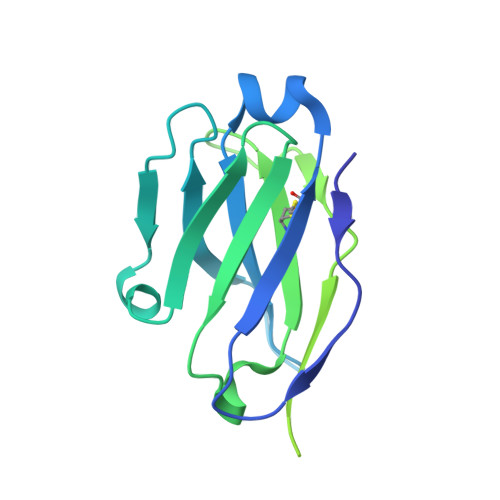
Structural basis for non-canonical integrin engagement by Bordetella adenylate cyclase toxin.
Goldsmith, J.A., DiVenere, A.M., Maynard, J.A., McLellan, J.S.(2022) Cell Rep 40: 111196-111196
- PubMed: 35977491
- DOI: https://doi.org/10.1016/j.celrep.2022.111196
- Primary Citation of Related Structures:
7USL, 7USM - PubMed Abstract:
Integrins are ubiquitous cell-surface heterodimers that are exploited by pathogens and toxins, including leukotoxins that target β 2 integrins on phagocytes. The Bordetella adenylate cyclase toxin (ACT) uses the α M β 2 integrin as a receptor, but the structural basis for integrin binding and neutralization by antibodies is poorly understood. Here, we use cryoelectron microscopy to determine a 2.7 Å resolution structure of an ACT fragment bound to α M β 2 . This structure reveals that ACT interacts with the headpiece and calf-2 of the α M subunit in a non-canonical manner specific to bent, inactive α M β 2 . Neutralizing antibody epitopes map to ACT residues involved in α M binding, providing the basis for antibody-mediated attachment inhibition. Furthermore, binding to α M β 2 positions the essential ACT acylation sites, which are conserved among toxins exported by type I secretion systems, at the cell membrane. These findings reveal a structural mechanism for integrin-mediated attachment and explain antibody-mediated neutralization of ACT intoxication.
- Department of Molecular Biosciences, The University of Texas at Austin, Austin, TX 78712, USA.
Organizational Affiliation: