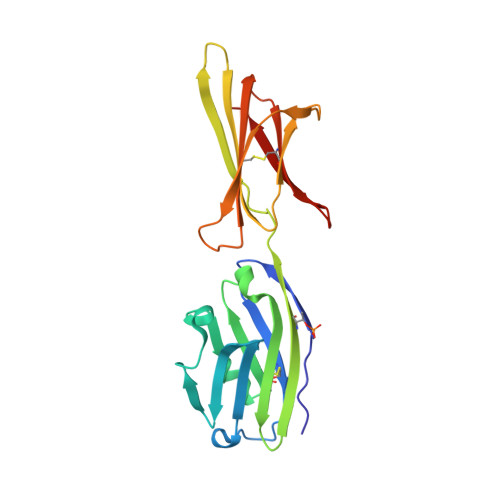
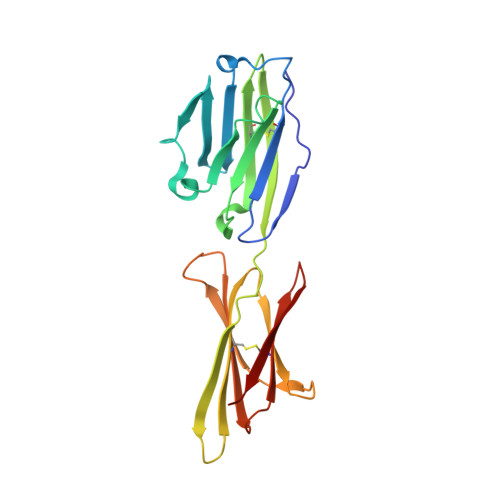
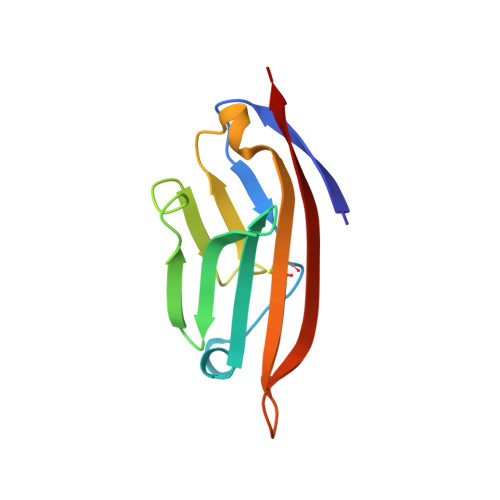The engineered CD80 variant fusion therapeutic davoceticept combines checkpoint antagonism with conditional CD28 costimulation for anti-tumor immunity.
Maurer, M.F., Lewis, K.E., Kuijper, J.L., Ardourel, D., Gudgeon, C.J., Chandrasekaran, S., Mudri, S.L., Kleist, K.N., Navas, C., Wolfson, M.F., Rixon, M.W., Swanson, R., Dillon, S.R., Levin, S.D., Kimbung, Y.R., Akutsu, M., Logan, D.T., Walse, B., Swiderek, K.M., Peng, S.L.(2022) Nat Commun 13: 1790-1790
- PubMed: 35379805
- DOI: https://doi.org/10.1038/s41467-022-29286-5
- Primary Citation of Related Structures:
7TPS - PubMed Abstract:
Despite the recent clinical success of T cell checkpoint inhibition targeting the CTLA-4 and PD-1 pathways, many patients either fail to achieve objective responses or they develop resistance to therapy. In some cases, poor responses to checkpoint blockade have been linked to suboptimal CD28 costimulation and the inability to generate and maintain a productive adaptive anti-tumor immune response. To address this, here we utilize directed evolution to engineer a CD80 IgV domain with increased PD-L1 affinity and fuse this to an immunoglobulin Fc domain, creating a therapeutic (ALPN-202, davoceticept) capable of providing CD28 costimulation in a PD-L1-dependent fashion while also antagonizing PD-1 - PD-L1 and CTLA-4-CD80/CD86 interactions. We demonstrate that by combining CD28 costimulation and dual checkpoint inhibition, ALPN-202 enhances T cell activation and anti-tumor efficacy in cell-based assays and mouse tumor models more potently than checkpoint blockade alone and thus has the potential to generate potent, clinically meaningful anti-tumor immunity in humans.
- Alpine Immune Sciences, Inc., Seattle, WA, USA. Mark.Maurer@AlpineImmuneSciences.com.
Organizational Affiliation: