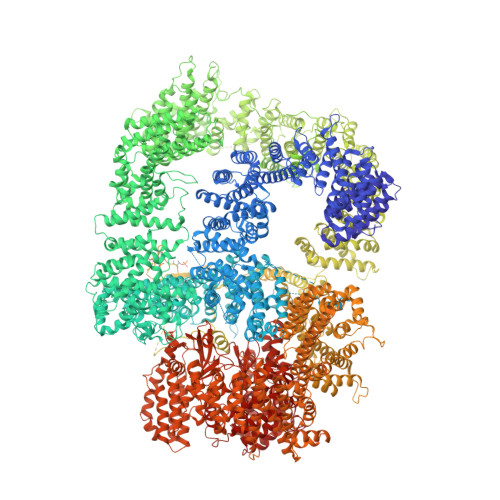
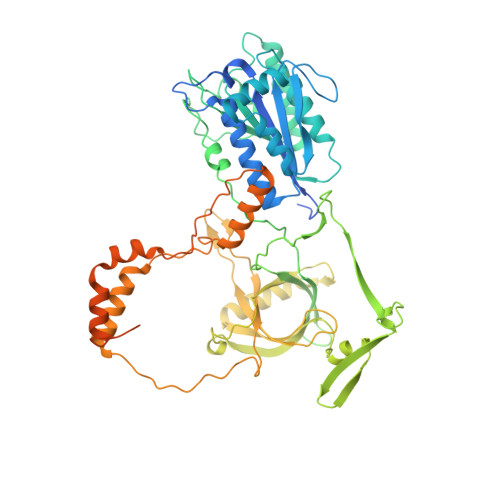
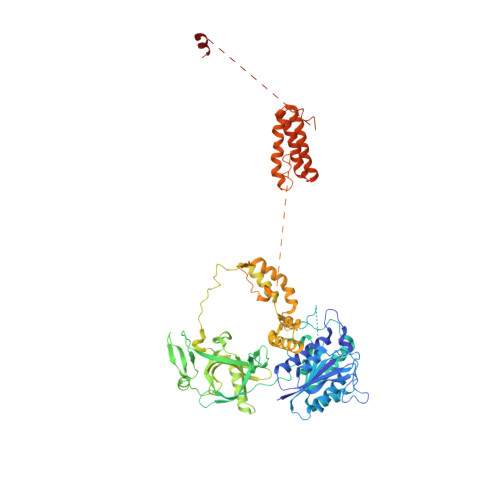
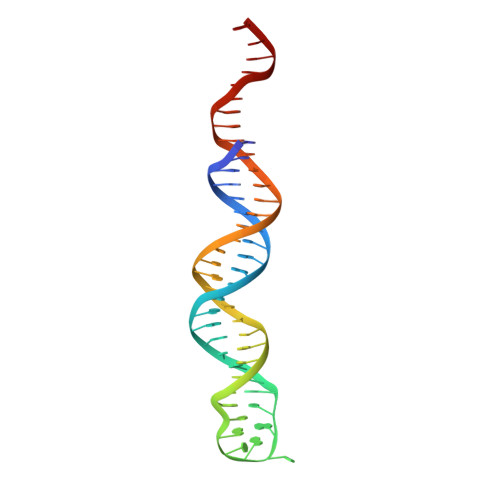
Autophosphorylation transforms DNA-PK from protecting to processing DNA ends.
Liu, L., Chen, X., Li, J., Wang, H., Buehl, C.J., Goff, N.J., Meek, K., Yang, W., Gellert, M.(2022) Mol Cell 82: 177
- PubMed: 34936881
- DOI: https://doi.org/10.1016/j.molcel.2021.11.025
- Primary Citation of Related Structures:
7SGL, 7SU3, 7SUD - PubMed Abstract:
The DNA-dependent protein kinase (DNA-PK) initially protects broken DNA ends but then promotes their processing during non-homologous end joining (NHEJ). Before ligation by NHEJ, DNA hairpin ends generated during V(D)J recombination must be opened by the Artemis nuclease, together with autophosphorylated DNA-PK. Structures of DNA-PK bound to DNA before and after phosphorylation, and in complex with Artemis and a DNA hairpin, reveal an essential functional switch. When bound to open DNA ends in its protection mode, DNA-PK is inhibited for cis-autophosphorylation of the so-called ABCDE cluster but activated for phosphorylation of other targets. In contrast, DNA hairpin ends promote cis-autophosphorylation. Phosphorylation of four Thr residues in ABCDE leads to gross structural rearrangement of DNA-PK, widening the DNA binding groove for Artemis recruitment and hairpin cleavage. Meanwhile, Artemis locks DNA-PK into the kinase-inactive state. Kinase activity and autophosphorylation of DNA-PK are regulated by different DNA ends, feeding forward to coordinate NHEJ events.
- Laboratory of Molecular Biology, NIDDK, National Institutes of Health, Bethesda, MD 20892, USA.
Organizational Affiliation: