Structure and Mechanism of a Cold-Adapted Bacterial Lipase
Lund, B.A., Svalberg, L., Purg, M., Chukwu, G., Widersten, M., Isaksen, G.V., Brandsdal, B.O., Aqvist, J.(2022) Biochemistry 61: 933-942
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(2022) Biochemistry 61: 933-942
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Lipase | 190 | Bacillus pumilus | Mutation(s): 0 Gene Names: L5 Membrane Entity: Yes | 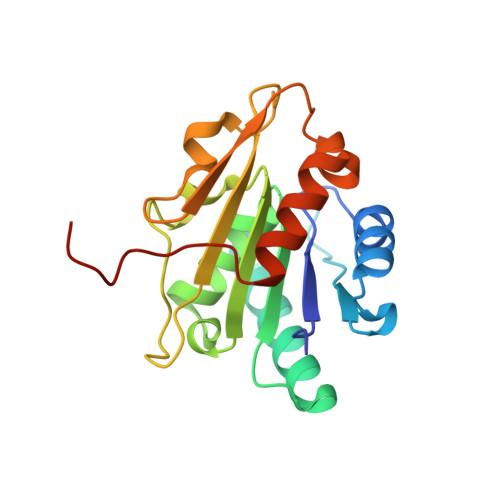 | |
UniProt | |||||
Find proteins for W8FKE7 (Bacillus pumilus) Explore W8FKE7 Go to UniProtKB: W8FKE7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | W8FKE7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CIT Query on CIT | B [auth A] | CITRIC ACID C6 H8 O7 KRKNYBCHXYNGOX-UHFFFAOYSA-N |  | ||
| PO4 Query on PO4 | C [auth A] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| PPI Query on PPI | D [auth A] | PROPANOIC ACID C3 H6 O2 XBDQKXXYIPTUBI-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 35.109 | α = 90 |
| b = 54.697 | β = 92.72 |
| c = 40.97 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Research Council of Norway | Norway | 262695 |
| Research Council of Norway | Norway | 274858 |