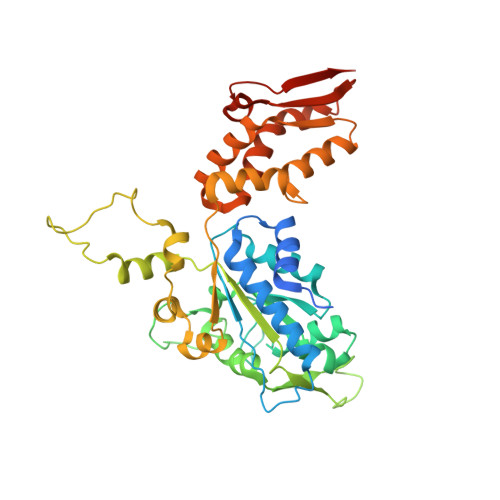
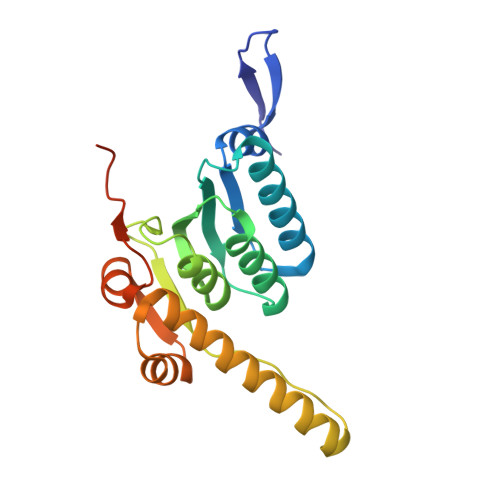
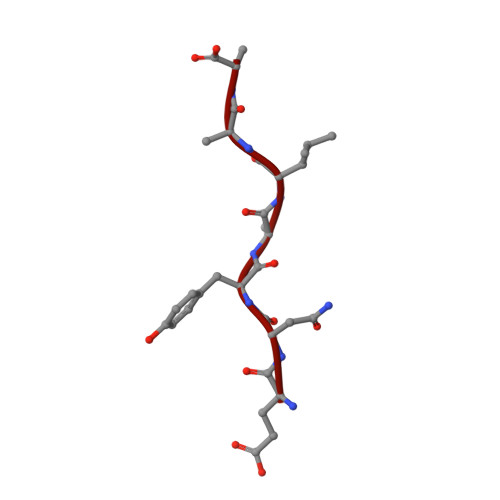
Structural basis of ClpXP recognition and unfolding of ssrA-tagged substrates.
Fei, X., Bell, T.A., Barkow, S.R., Baker, T.A., Sauer, R.T.(2020) Elife 9
- PubMed: 33089779
- DOI: https://doi.org/10.7554/eLife.61496
- Primary Citation of Related Structures:
6WR2, 6WRF, 6WSG - PubMed Abstract:
When ribosomes fail to complete normal translation, all cells have mechanisms to ensure degradation of the resulting partial proteins to safeguard proteome integrity. In Escherichia coli and other eubacteria, the tmRNA system rescues stalled ribosomes and adds an ssrA tag or degron to the C-terminus of the incomplete protein, which directs degradation by the AAA+ ClpXP protease. Here, we present cryo-EM structures of ClpXP bound to the ssrA degron. C-terminal residues of the ssrA degron initially bind in the top of an otherwise closed ClpX axial channel and subsequently move deeper into an open channel. For short-degron protein substrates, we show that unfolding can occur directly from the initial closed-channel complex. For longer degron substrates, our studies illuminate how ClpXP transitions from specific recognition into a nonspecific unfolding and translocation machine. Many AAA+ proteases and protein-remodeling motors are likely to employ similar multistep recognition and engagement strategies.
- Departments of Biology, Massachusetts Institute of Technology, Cambridge, United States.
Organizational Affiliation: