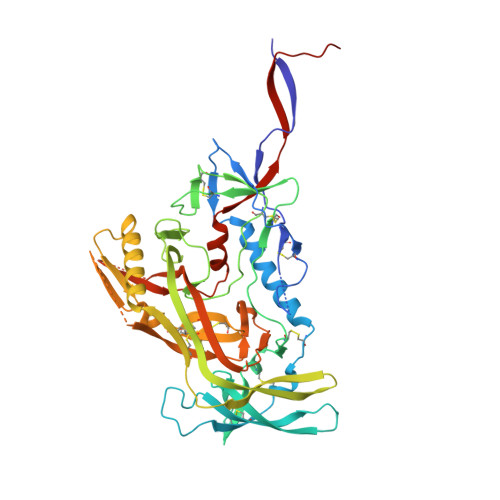
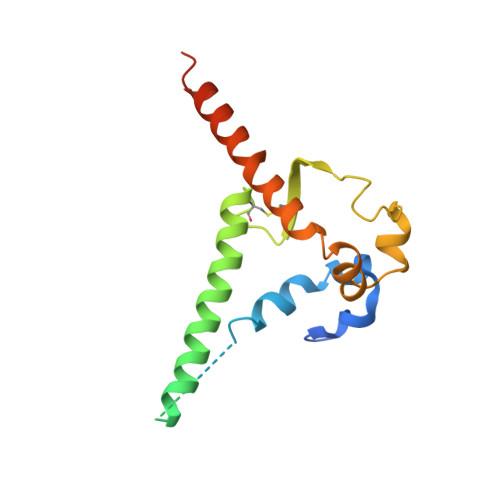
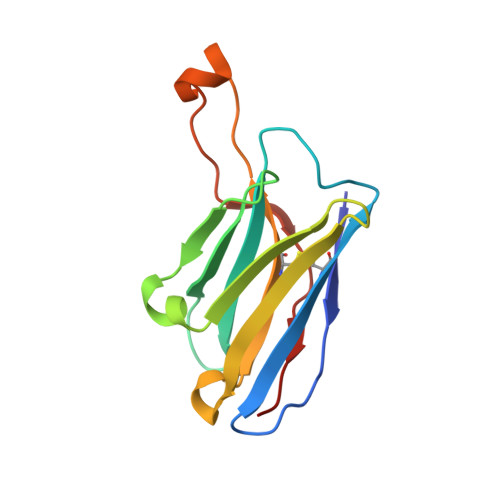
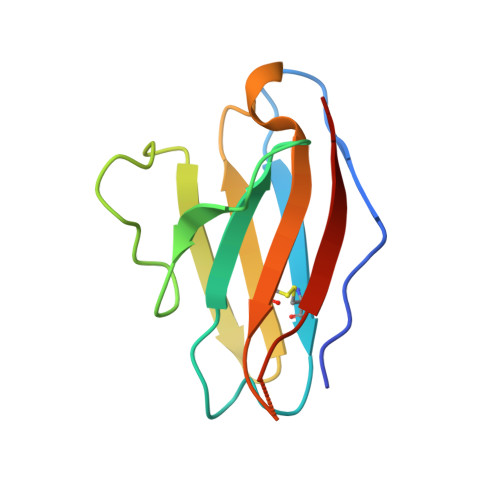
A generalized HIV vaccine design strategy for priming of broadly neutralizing antibody responses.
Steichen, J.M., Lin, Y.C., Havenar-Daughton, C., Pecetta, S., Ozorowski, G., Willis, J.R., Toy, L., Sok, D., Liguori, A., Kratochvil, S., Torres, J.L., Kalyuzhniy, O., Melzi, E., Kulp, D.W., Raemisch, S., Hu, X., Bernard, S.M., Georgeson, E., Phelps, N., Adachi, Y., Kubitz, M., Landais, E., Umotoy, J., Robinson, A., Briney, B., Wilson, I.A., Burton, D.R., Ward, A.B., Crotty, S., Batista, F.D., Schief, W.R.(2019) Science 366
- PubMed: 31672916
- DOI: https://doi.org/10.1126/science.aax4380
- Primary Citation of Related Structures:
6DFG, 6DFH, 6NF5, 6NFC, 6OC7 - PubMed Abstract:
Vaccine induction of broadly neutralizing antibodies (bnAbs) to HIV remains a major challenge. Germline-targeting immunogens hold promise for initiating the induction of certain bnAb classes; yet for most bnAbs, a strong dependence on antibody heavy chain complementarity-determining region 3 (HCDR3) is a major barrier. Exploiting ultradeep human antibody sequencing data, we identified a diverse set of potential antibody precursors for a bnAb with dominant HCDR3 contacts. We then developed HIV envelope trimer-based immunogens that primed responses from rare bnAb-precursor B cells in a mouse model and bound a range of potential bnAb-precursor human naïve B cells in ex vivo screens. Our repertoire-guided germline-targeting approach provides a framework for priming the induction of many HIV bnAbs and could be applied to most HCDR3-dominant antibodies from other pathogens.
- Department of Immunology and Microbiology, The Scripps Research Institute, La Jolla, CA 92037, USA.
Organizational Affiliation: