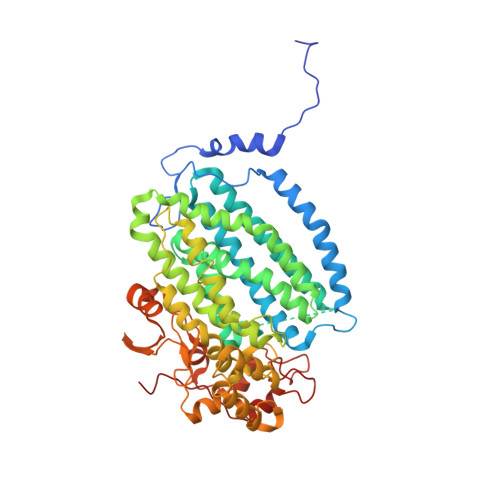
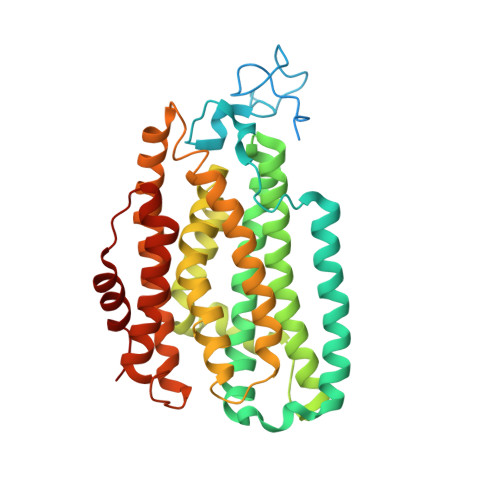
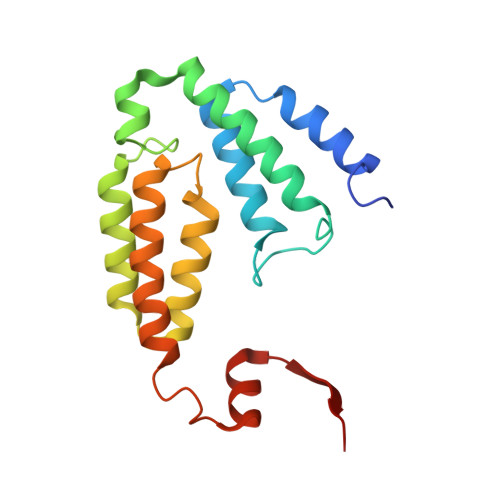
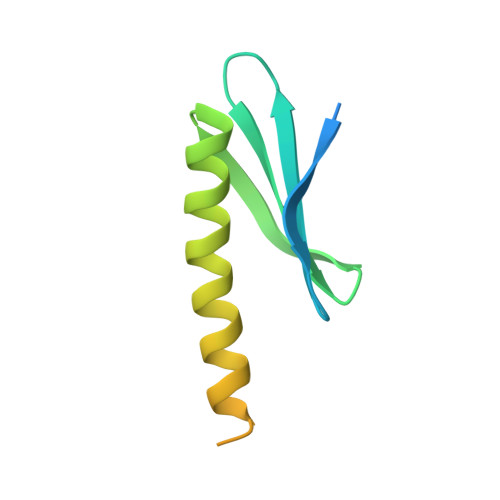
MMOD-induced structural changes of hydroxylase in soluble methane monooxygenase.
Kim, H., An, S., Park, Y.R., Jang, H., Yoo, H., Park, S.H., Lee, S.J., Cho, U.S.(2019) Sci Adv 5: eaax0059-eaax0059
- PubMed: 31616787
- DOI: https://doi.org/10.1126/sciadv.aax0059
- Primary Citation of Related Structures:
6D7K - PubMed Abstract:
Soluble methane monooxygenase in methanotrophs converts methane to methanol under ambient conditions. The maximum catalytic activity of hydroxylase (MMOH) is achieved through the interplay of its regulatory protein (MMOB) and reductase. An additional auxiliary protein, MMOD, functions as an inhibitor of MMOH; however, its inhibitory mechanism remains unknown. Here, we report the crystal structure of the MMOH-MMOD complex from Methylosinus sporium strain 5 (2.6 Å). Its structure illustrates that MMOD associates with the canyon region of MMOH where MMOB binds. Although MMOD and MMOB recognize the same binding site, each binding component triggers different conformational changes toward MMOH, which then respectively lead to the inhibition and activation of MMOH. Particularly, MMOD binding perturbs the di-iron geometry by inducing two major MMOH conformational changes, i.e., MMOH β subunit disorganization and subsequent His 147 dissociation with Fe1 coordination. Furthermore, 1,6-hexanediol, a mimic of the products of sMMO, reveals the substrate access route.
- Department of Biological Chemistry, University of Michigan, Ann Arbor, MI 48109, USA.
Organizational Affiliation: