Structural insights into DNA degradation by human mitochondrial nuclease MGME1
Yang, C., Wu, R., Liu, H., Chen, Y., Gao, Y., Chen, X., Li, Y., Ma, J., Li, J., Gan, J.(2018) Nucleic Acids Res 46: 11075-11088
- PubMed: 30247721
- DOI: https://doi.org/10.1093/nar/gky855
- Primary Citation of Related Structures:
5ZYT, 5ZYU, 5ZYV, 5ZYW - PubMed Abstract:
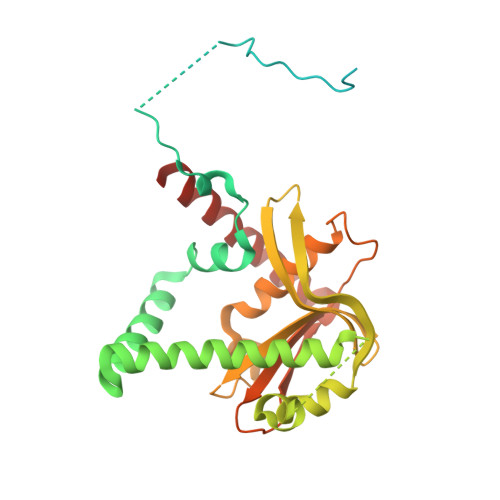
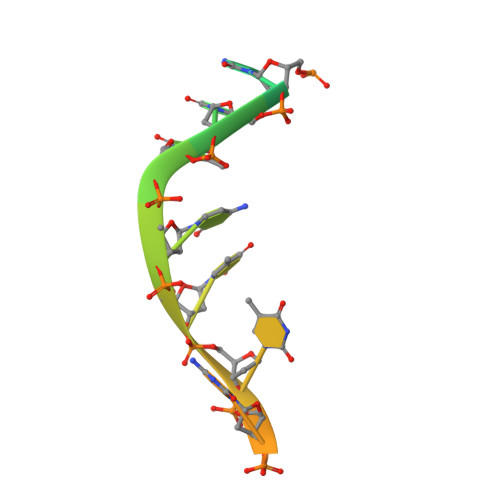
Mitochondrial nucleases play important roles in accurate maintenance and correct metabolism of mtDNA, the own genetic materials of mitochondria that are passed exclusively from mother to child. MGME1 is a highly conserved DNase that was discovered recently. Mutations in MGME1-coding gene lead to severe mitochondrial syndromes characterized by external ophthalmoplegia, emaciation, and respiratory failure in humans. Unlike many other nucleases that are distributed in multiple cellular organelles, human MGME1 is a mitochondria-specific nuclease; therefore, it can serve as an ideal target for treating related syndromes. Here, we report one HsMGME1-Mn2+ complex and three different HsMGME1-DNA complex structures. In combination with in vitro cleavage assays, our structures reveal the detailed molecular basis for substrate DNA binding and/or unwinding by HsMGME1. Besides the conserved two-cation-assisted catalytic mechanism, structural analysis of HsMGME1 and comparison with homologous proteins also clarified substrate binding and cleavage directionalities of the DNA double-strand break repair complexes RecBCD and AddAB.
- State Key Laboratory of Genetic Engineering, Collaborative Innovation Center of Genetics and Development, Department of Physiology and Biophysics, School of Life Sciences, Fudan University, Shanghai 200433, China.
Organizational Affiliation: