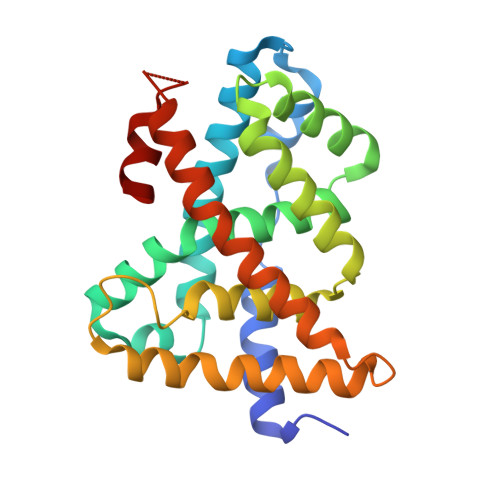
D3R Grand Challenge 2: blind prediction of protein-ligand poses, affinity rankings, and relative binding free energies.
Gaieb, Z., Liu, S., Gathiaka, S., Chiu, M., Yang, H., Shao, C., Feher, V.A., Walters, W.P., Kuhn, B., Rudolph, M.G., Burley, S.K., Gilson, M.K., Amaro, R.E.(2018) J Comput Aided Mol Des 32: 1-20
- PubMed: 29204945
- DOI: https://doi.org/10.1007/s10822-017-0088-4
- Primary Citation of Related Structures:
5Q0I, 5Q0J, 5Q0K, 5Q0L, 5Q0M, 5Q0N, 5Q0O, 5Q0P, 5Q0Q, 5Q0R, 5Q0S, 5Q0T, 5Q0U, 5Q0V, 5Q0W, 5Q0X, 5Q0Y, 5Q0Z, 5Q10, 5Q11, 5Q12, 5Q13, 5Q14, 5Q15, 5Q16, 5Q17, 5Q18, 5Q19, 5Q1A, 5Q1B, 5Q1C, 5Q1D, 5Q1E, 5Q1F, 5Q1G, 5Q1H, 5Q1I - PubMed Abstract:
The Drug Design Data Resource (D3R) ran Grand Challenge 2 (GC2) from September 2016 through February 2017. This challenge was based on a dataset of structures and affinities for the nuclear receptor farnesoid X receptor (FXR), contributed by F. Hoffmann-La Roche. The dataset contained 102 IC50 values, spanning six orders of magnitude, and 36 high-resolution co-crystal structures with representatives of four major ligand classes. Strong global participation was evident, with 49 participants submitting 262 prediction submission packages in total. Procedurally, GC2 mimicked Grand Challenge 2015 (GC2015), with a Stage 1 subchallenge testing ligand pose prediction methods and ranking and scoring methods, and a Stage 2 subchallenge testing only ligand ranking and scoring methods after the release of all blinded co-crystal structures. Two smaller curated sets of 18 and 15 ligands were developed to test alchemical free energy methods. This overview summarizes all aspects of GC2, including the dataset details, challenge procedures, and participant results. We also consider implications for progress in the field, while highlighting methodological areas that merit continued development. Similar to GC2015, the outcome of GC2 underscores the pressing need for methods development in pose prediction, particularly for ligand scaffolds not currently represented in the Protein Data Bank ( http://www.pdb.org ), and in affinity ranking and scoring of bound ligands.
- Drug Design Data Resource, University of California, San Diego, La Jolla, CA, 92093, USA.
Organizational Affiliation: