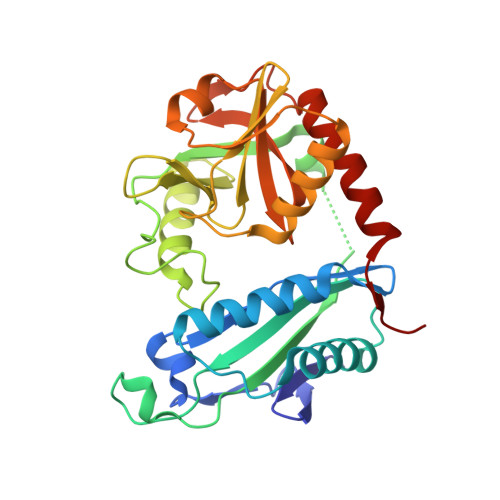
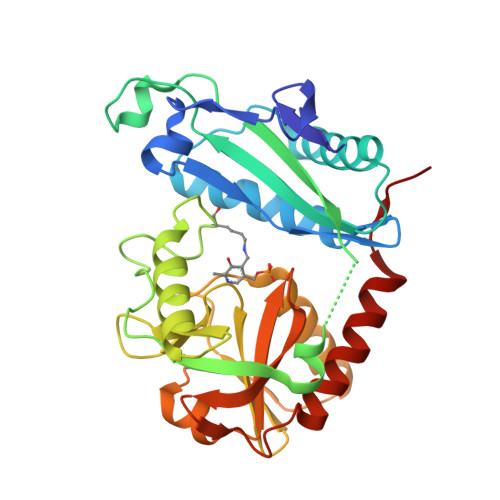
Thermostable Branched-Chain Amino Acid Transaminases From the ArchaeaGeoglobus acetivoransandArchaeoglobus fulgidus: Biochemical and Structural Characterization.
Isupov, M.N., Boyko, K.M., Sutter, J.M., James, P., Sayer, C., Schmidt, M., Schonheit, P., Nikolaeva, A.Y., Stekhanova, T.N., Mardanov, A.V., Ravin, N.V., Bezsudnova, E.Y., Popov, V.O., Littlechild, J.A.(2019) Front Bioeng Biotechnol 7: 7-7
- PubMed: 30733943
- DOI: https://doi.org/10.3389/fbioe.2019.00007
- Primary Citation of Related Structures:
5CM0, 5E25, 5MQZ, 5MR0 - PubMed Abstract:
Two new thermophilic branched chain amino acid transaminases have been identified within the genomes of different hyper-thermophilic archaea, Geoglobus acetivorans , and Archaeoglobus fulgidus . These enzymes belong to the class IV of transaminases as defined by their structural fold. The enzymes have been cloned and over-expressed in Escherichia coli and the recombinant enzymes have been characterized both biochemically and structurally. Both enzymes showed high thermostability with optimal temperature for activity at 80 and 85°C, respectively. They retain good activity after exposure to 50% of the organic solvents, ethanol, methanol, DMSO and acetonitrile. The enzymes show a low activity to ( R )-methylbenzylamine but no activity to ( S )-methylbenzylamine. Both enzymes have been crystallized and their structures solved in the internal aldimine form, to 1.9 Å resolution for the Geoglobus enzyme and 2.0 Å for the Archaeoglobus enzyme. Also the Geoglobus enzyme structure has been determined in complex with the amino acceptor α-ketoglutarate and the Archaeoglobus enzyme in complex with the inhibitor gabaculine. These two complexes have helped to determine the conformation of the enzymes during enzymatic turnover and have increased understanding of their substrate specificity. A comparison has been made with another ( R ) selective class IV transaminase from the fungus Nectria haematococca which was previously studied in complex with gabaculine. The subtle structural differences between these enzymes has provided insight regarding their different substrate specificities.
- Henry Wellcome Building for Biocatalysis, Biosciences, University of Exeter, Exeter, United Kingdom.
Organizational Affiliation: