Discovery and Design of novel Tubulin inhibitors
Zhang, H., Luo, C.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Tubulin alpha-1B chain | 440 | Bos taurus | Mutation(s): 0 EC: 3.6.5 | 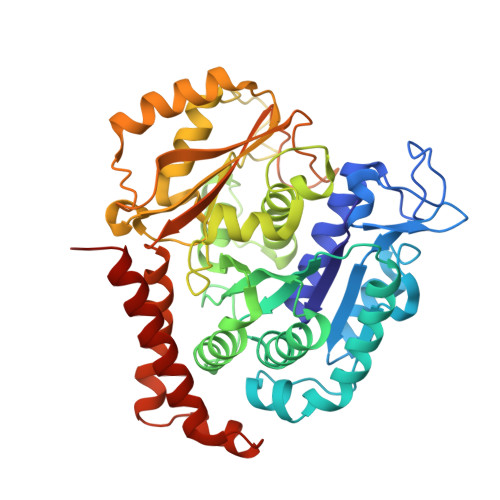 | |
UniProt | |||||
Find proteins for P81947 (Bos taurus) Explore P81947 Go to UniProtKB: P81947 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P81947 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Tubulin beta-2B chain | 431 | Bos taurus | Mutation(s): 0 | 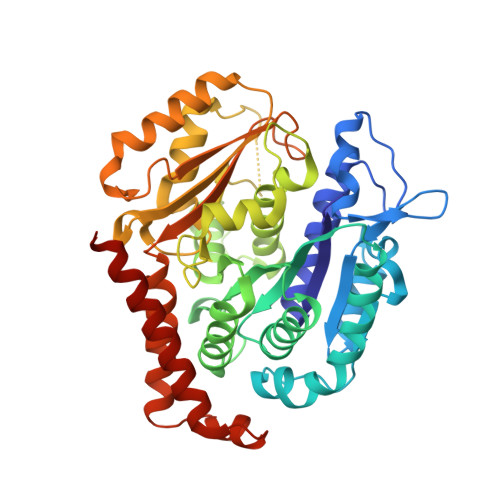 | |
UniProt | |||||
Find proteins for Q6B856 (Bos taurus) Explore Q6B856 Go to UniProtKB: Q6B856 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q6B856 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Stathmin-4 | 185 | Rattus norvegicus | Mutation(s): 0 | 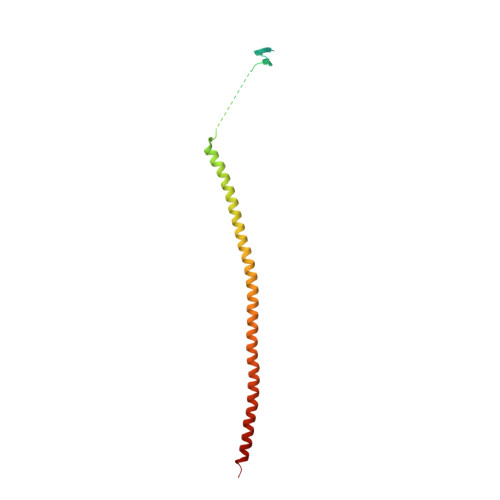 | |
UniProt | |||||
Find proteins for P63043 (Rattus norvegicus) Explore P63043 Go to UniProtKB: P63043 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P63043 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Tubulin tyrosine ligase | 378 | Gallus gallus | Mutation(s): 0 | 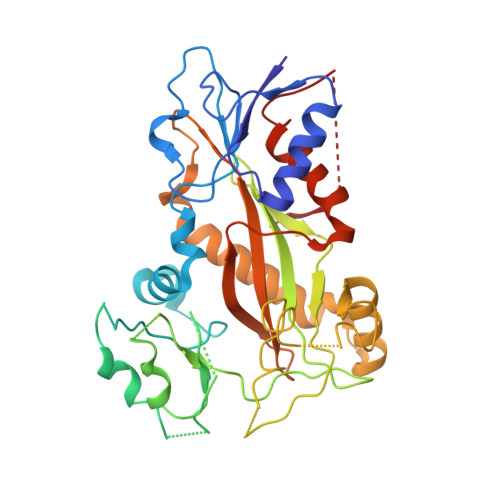 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 9 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GTP Query on GTP | G [auth A], U [auth C] | GUANOSINE-5'-TRIPHOSPHATE C10 H16 N5 O14 P3 XKMLYUALXHKNFT-UUOKFMHZSA-N |  | ||
| ACP Query on ACP | HA [auth F] | PHOSPHOMETHYLPHOSPHONIC ACID ADENYLATE ESTER C11 H18 N5 O12 P3 UFZTZBNSLXELAL-IOSLPCCCSA-N |  | ||
| GDP Query on GDP | DA [auth D], M [auth B] | GUANOSINE-5'-DIPHOSPHATE C10 H15 N5 O11 P2 QGWNDRXFNXRZMB-UUOKFMHZSA-N |  | ||
| 97O Query on 97O | R [auth B] | 6,7,8-trimethoxy-1-(4-methoxyphenyl)-4,5-dihydro-2~{H}-benzo[e]indazole C21 H22 N2 O4 LOJZGYGSTOWCFA-UHFFFAOYSA-N |  | ||
| MES Query on MES | Q [auth B] | 2-(N-MORPHOLINO)-ETHANESULFONIC ACID C6 H13 N O4 S SXGZJKUKBWWHRA-UHFFFAOYSA-N |  | ||
| GOL Query on GOL | FA [auth D] GA [auth D] I [auth A] J [auth A] L [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| IMD Query on IMD | AA [auth C], BA [auth C], CA [auth C] | IMIDAZOLE C3 H5 N2 RAXXELZNTBOGNW-UHFFFAOYSA-O |  | ||
| CA Query on CA | K [auth A], P [auth B], Z [auth C] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| MG Query on MG | EA [auth D], H [auth A], N [auth B], V [auth C] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 103.832 | α = 90 |
| b = 154.843 | β = 90 |
| c = 181.024 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |