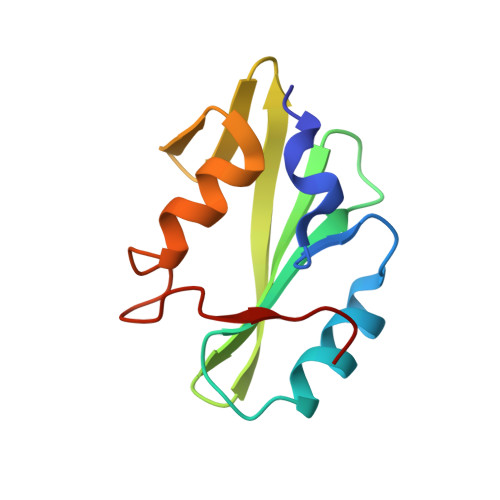
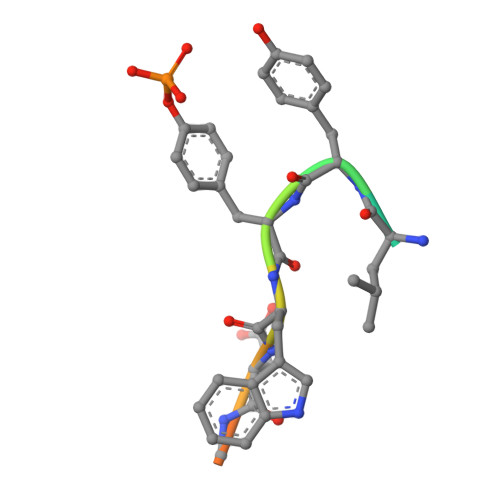
Multimodal Recognition of Diverse Peptides by the C-Terminal SH2 Domain of Phospholipase C-gamma 1 Protein.
McKercher, M.A., Guan, X., Tan, Z., Wuttke, D.S.(2017) Biochemistry 56: 2225-2237
- PubMed: 28376302
- DOI: https://doi.org/10.1021/acs.biochem.7b00023
- Primary Citation of Related Structures:
5TNW, 5TO4, 5TQ1, 5TQS - PubMed Abstract:
SH2 domains recognize phosphotyrosine (pY)-containing peptide ligands and play key roles in the regulation of receptor tyrosine kinase pathways. Each SH2 domain has individualized specificity, encoded in the amino acids neighboring the pY, for defined targets that convey their distinct functions. The C-terminal SH2 domain (PLCC) of the phospholipase C-γ1 full-length protein (PLCγ1) typically binds peptides containing small and hydrophobic amino acids adjacent to the pY, including a peptide derived from platelet-derived growth factor receptor B (PDGFRB) and an intraprotein recognition site (Y783 of PLCγ1) involved in the regulation of the protein's lipase activity. Remarkably, PLCC also recognizes unexpected peptides containing amino acids with polar or bulky side chains that deviate from this pattern. This versatility in recognition specificity may allow PLCγ1 to participate in diverse, previously unrecognized, signaling pathways in response to binding chemically dissimilar partners. We have used structural approaches, including nuclear magnetic resonance and X-ray crystallography, to elucidate the mechanisms of noncognate peptide binding to PLCC by ligands derived from receptor tyrosine kinase ErbB2 and from the insulin receptor. The high-resolution peptide-bound structures reveal that PLCC has a relatively static backbone but contains a chemically rich protein surface comprised of a combination of hydrophobic pockets and amino acids with charged side chains. We demonstrate that this expansive and chemically diverse PLCC interface, in addition to peptide conformational plasticity, permits PLCC to recognize specific noncognate peptide ligands with multimodal specificity.
- Department of Chemistry and Biochemistry, University of Colorado Boulder , Boulder, Colorado 80309, United States.
Organizational Affiliation: