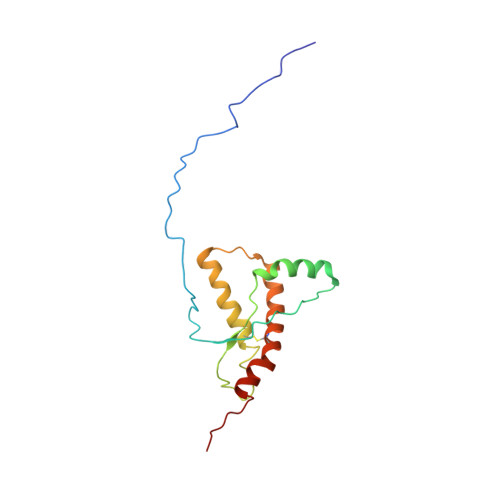
Truncated prion protein PrP226* - A structural view on its role in amyloid disease.
Kovac, V., Zupancic, B., Ilc, G., Plavec, J., Curin Serbec, V.(2017) Biochem Biophys Res Commun 484: 45-50
- PubMed: 28109886
- DOI: https://doi.org/10.1016/j.bbrc.2017.01.078
- Primary Citation of Related Structures:
5L6R - PubMed Abstract:
In the brain of patients with transmissible spongiform encephalopathies, besides PrP Sc aggregates, deposition of truncated PrP molecules was described. Jansen et al. reported two clinical cases with deposition of C-terminally truncated PrP, one of them ending with Tyr226. We have previously described the discovery of monoclonal antibody V5B2 that selectively recognizes this version of the prion protein, which we called PrP226*. Using monoclonal antibody V5B2 we showed that accumulation of PrP226* is characteristic for most types of human and animal TSEs. Its distribution correlates to the distribution of PrP Sc aggregates. To gain insight into the structural basis of its presence and distribution in PrP aggregates, we have determined the NMR structure of recombinant PrP226*. The structure of the protein consists of a disordered N-terminal part (residues 90-125) and a structured C-terminal part (residues 126-226). The C-terminal segment consists of four α-helices and a short antiparallel β-sheet. Our model predicts a break in the C-terminal helix and reorganized hydrophobic interactions between helix α 3 and β 2 -α 2 loop due to the shorter C-terminus. The structural model gives information on the possible role of the protein in the development of amyloid disease and can serve as a foundation to develop tools for prevention and treatment of prion diseases.
- Department for the Production of Diagnostic Reagents and Research & R&D Service, Blood Transfusion Centre of Slovenia, Šlajmerjeva 6, SI-1000 Ljubljana, Slovenia. Electronic address: valerija.kovac@ztm.si.
Organizational Affiliation: