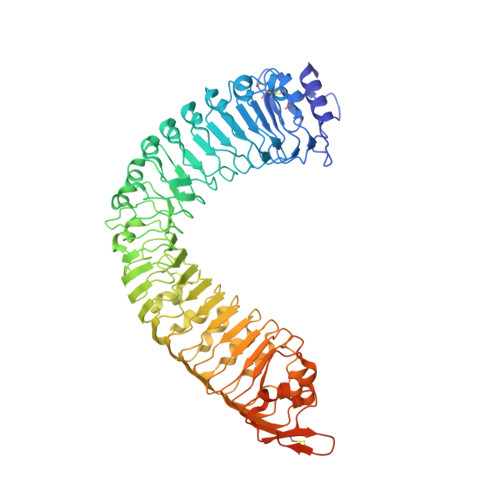
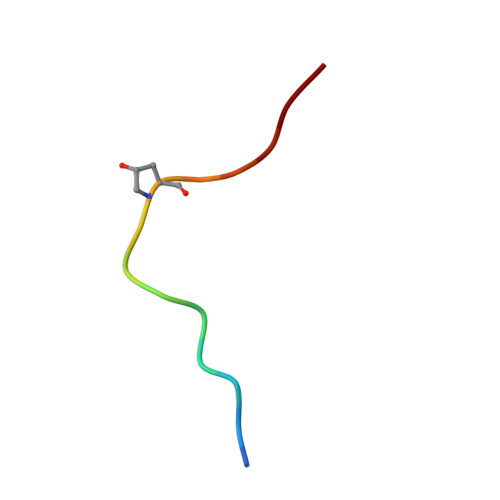
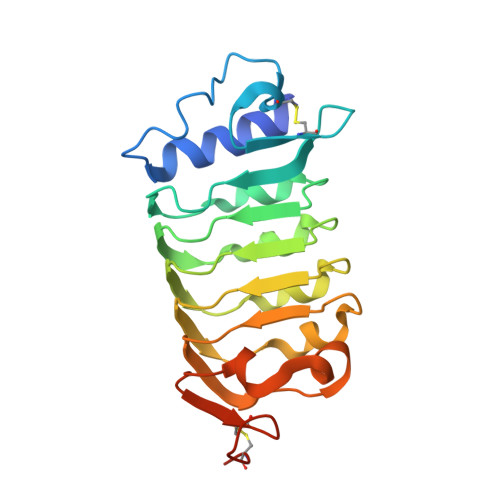
Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission.
Santiago, J., Brandt, B., Wildhagen, M., Hohmann, U., Hothorn, L.A., Butenko, M.A., Hothorn, M.(2016) Elife 5
- PubMed: 27058169
- DOI: https://doi.org/10.7554/eLife.15075
- Primary Citation of Related Structures:
5IXO, 5IXQ, 5IXT, 5IYV, 5IYX - PubMed Abstract:
Plants constantly renew during their life cycle and thus require to shed senescent and damaged organs. Floral abscission is controlled by the leucine-rich repeat receptor kinase (LRR-RK) HAESA and the peptide hormone IDA. It is unknown how expression of IDA in the abscission zone leads to HAESA activation. Here we show that IDA is sensed directly by the HAESA ectodomain. Crystal structures of HAESA in complex with IDA reveal a hormone binding pocket that accommodates an active dodecamer peptide. A central hydroxyproline residue anchors IDA to the receptor. The HAESA co-receptor SERK1, a positive regulator of the floral abscission pathway, allows for high-affinity sensing of the peptide hormone by binding to an Arg-His-Asn motif in IDA. This sequence pattern is conserved among diverse plant peptides, suggesting that plant peptide hormone receptors may share a common ligand binding mode and activation mechanism.
- Structural Plant Biology Laboratory, Department of Botany and Plant Biology, University of Geneva, Geneva, Switzerland.
Organizational Affiliation: