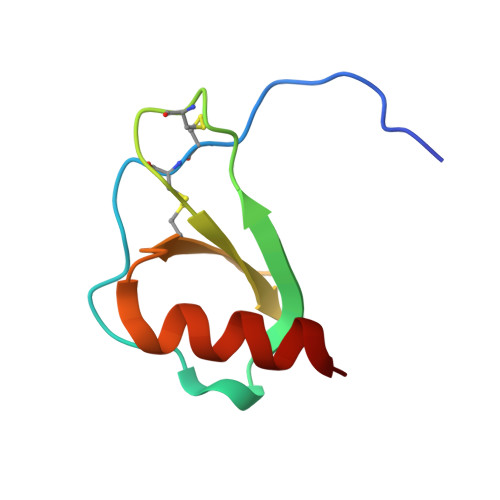
Structures of human CCL18, CCL3, and CCL4 reveal molecular determinants for quaternary structures and sensitivity to insulin-degrading enzyme.
Liang, W.G., Ren, M., Zhao, F., Tang, W.J.(2015) J Mol Biology 427: 1345-1358
- PubMed: 25636406
- DOI: https://doi.org/10.1016/j.jmb.2015.01.012
- Primary Citation of Related Structures:
3TN2, 4MHE, 4RA8, 4RAL - PubMed Abstract:
CC chemokine ligands (CCLs) are 8- to 14-kDa signaling proteins involved in diverse immune functions. While CCLs share similar tertiary structures, oligomerization produces highly diverse quaternary structures that protect chemokines from proteolytic degradation and modulate their functions. CCL18 is closely related to CCL3 and CCL4 with respect to both protein sequence and genomic location, yet CCL18 has distinct biochemical and biophysical properties. Here, we report a crystal structure of human CCL18 and its oligomerization states in solution based on crystallographic and small-angle X-ray scattering analyses. Our data show that CCL18 adopts an α-helical conformation at its N-terminus that weakens its dimerization, explaining CCL18's preference for the monomeric state. Multiple contacts between monomers allow CCL18 to reversibly form a unique open-ended oligomer different from those of CCL3, CCL4, and CCL5. Furthermore, these differences hinge on proline 8, which is conserved in CCL3 and CCL4 but is replaced by lysine in human CCL18. Our structural analyses suggest that a mutation of proline 8 to alanine stabilizes a type 1 β-turn at the N-terminus of CCL4 to prevent dimerization but prevents dimers from making key contacts with each other in CCL3. Thus, the P8A mutation induces depolymerization of CCL3 and CCL4 by distinct mechanisms. Finally, we used structural, biochemical, and functional analyses to unravel why insulin-degrading enzyme degrades CCL3 and CCL4 but not CCL18. Our results elucidate the molecular basis for the oligomerization of three closely related CC chemokines and suggest how oligomerization shapes CCL chemokine function.
- Ben May Department for Cancer Research, The University of Chicago, IL 60637, USA.
Organizational Affiliation: