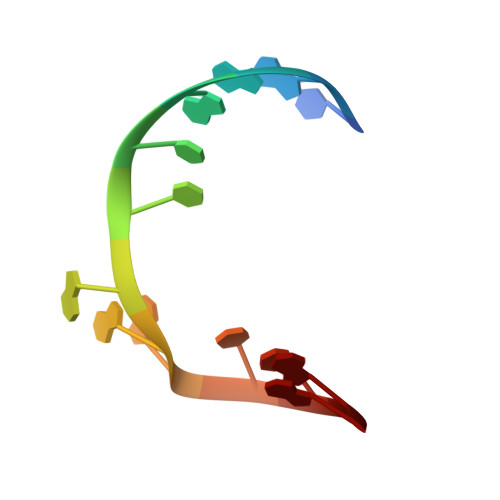
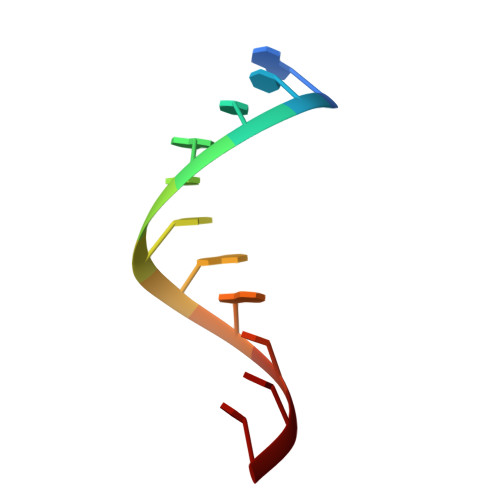
Crystal structure of a lead-dependent ribozyme revealing metal binding sites relevant to catalysis.
Wedekind, J.E., McKay, D.B.(1999) Nat Struct Biol 6: 261-268
- PubMed: 10074945
- DOI: https://doi.org/10.1038/6700
- Primary Citation of Related Structures:
429D - PubMed Abstract:
The leadzyme is a small RNA motif that catalyzes a site-specific, Pb2+-dependent cleavage reaction. As such, it is an example of a metal-dependent RNA enzyme. Here we describe the X-ray crystallographic structure of the leadzyme, which reveals two independent molecules per asymmetric unit. Both molecules feature an internal loop in which a bulged purine base stack twists away from the helical stem. This kinks the backbone, rendering the phosphodiester bond susceptible to cleavage. The independent molecules have different conformations: one leadzyme copy coordinates Mg2+, whereas the other binds only Ba2+ or Pb2+. In the active site of the latter molecule, a single Ba2+ ion coordinates the 2'-OH nucleophile, and appears to mimic the binding of catalytic lead. These observations allow a bond cleavage reaction to be modeled, which reveals the minimal structural features necessary for catalysis by this small ribozyme.
- Department of Structural Biology, Stanford University School of Medicine, California 94305-5126, USA. Wedekind@ribose.stanford.edu
Organizational Affiliation: