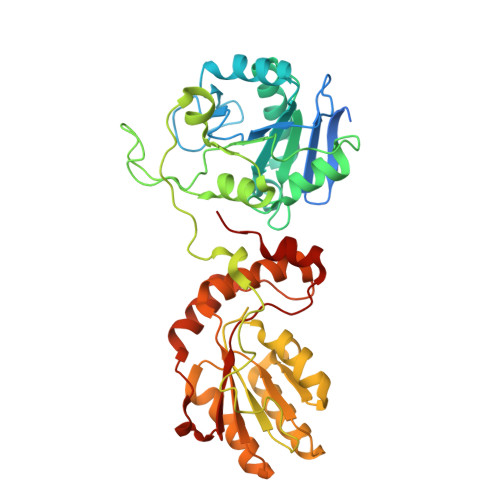
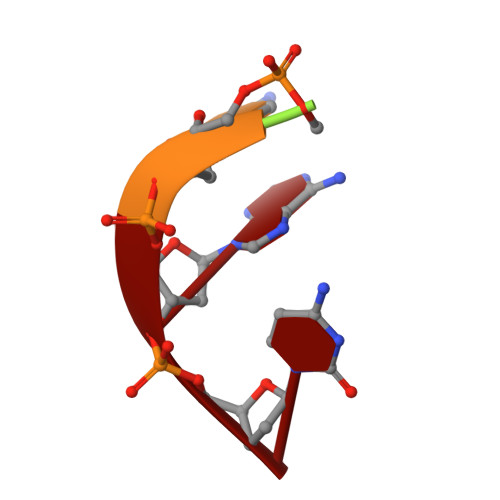
The structural basis for substrate recognition by mammalian polynucleotide kinase 3' phosphatase.
Garces, F., Pearl, L.H., Oliver, A.W.(2011) Mol Cell 44: 385-396
- PubMed: 22055185
- DOI: https://doi.org/10.1016/j.molcel.2011.08.036
- Primary Citation of Related Structures:
3ZVL, 3ZVM, 3ZVN - PubMed Abstract:
Mammalian polynucleotide kinase 3' phosphatase (PNK) plays a key role in the repair of DNA damage, functioning as part of both the nonhomologous end-joining (NHEJ) and base excision repair (BER) pathways. Through its two catalytic activities, PNK ensures that DNA termini are compatible with extension and ligation by either removing 3'-phosphates from, or by phosphorylating 5'-hydroxyl groups on, the ribose sugar of the DNA backbone. We have now determined crystal structures of murine PNK with DNA molecules bound to both of its active sites. The structure of ssDNA engaged with the 3'-phosphatase domain suggests a mechanism of substrate interaction that assists DNA end seeking. The structure of dsDNA bound to the 5'-kinase domain reveals a mechanism of DNA bending that facilitates recognition of DNA ends in the context of single-strand and double-strand breaks and suggests a close functional cooperation in substrate recognition between the kinase and phosphatase active sites.
- Cancer Research UK DNA Repair Enzymes Group, Genome Damage and Stability Centre, School of Life Sciences, University of Sussex, Falmer BN1 9QG, UK.
Organizational Affiliation: