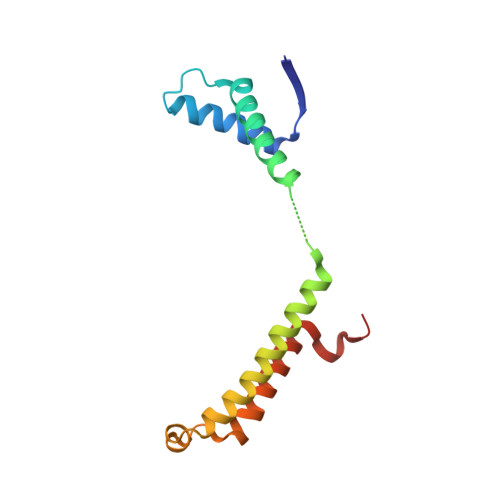
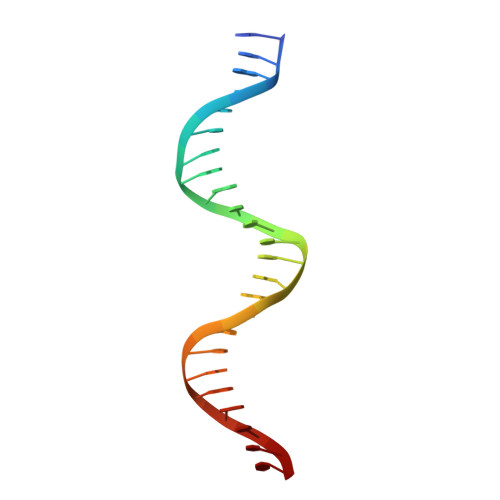
Structural basis of cooperative DNA recognition by the plasmid conjugation factor, TraM.
Wong, J.J., Lu, J., Edwards, R.A., Frost, L.S., Glover, J.N.(2011) Nucleic Acids Res 39: 6775-6788
- PubMed: 21565799
- DOI: https://doi.org/10.1093/nar/gkr296
- Primary Citation of Related Structures:
3OMY, 3ON0 - PubMed Abstract:
The conjugative transfer of F-like plasmids such as F, R1, R100 and pED208, between bacterial cells requires TraM, a plasmid-encoded DNA-binding protein. TraM tetramers bridge the origin of transfer (oriT) to a key component of the conjugative pore, the coupling protein TraD. Here we show that TraM recognizes a high-affinity DNA-binding site, sbmA, as a cooperative dimer of tetramers. The crystal structure of the TraM-sbmA complex from the plasmid pED208 shows that binding cooperativity is mediated by DNA kinking and unwinding, without any direct contact between tetramers. Sequence-specific DNA recognition is carried out by TraM's N-terminal ribbon-helix-helix (RHH) domains, which bind DNA in a staggered arrangement. We demonstrate that both DNA-binding specificity, as well as selective interactions between TraM and the C-terminal tail of its cognate TraD mediate conjugation specificity within the F-like family of plasmids. The ability of TraM to cooperatively bind DNA without interaction between tetramers leaves the C-terminal TraM tetramerization domains free to make multiple interactions with TraD, driving recruitment of the plasmid to the conjugative pore.
- Department of Biochemistry, School of Molecular and Systems Medicine, University of Alberta, Edmonton, AB T6G 2H7, Canada.
Organizational Affiliation: