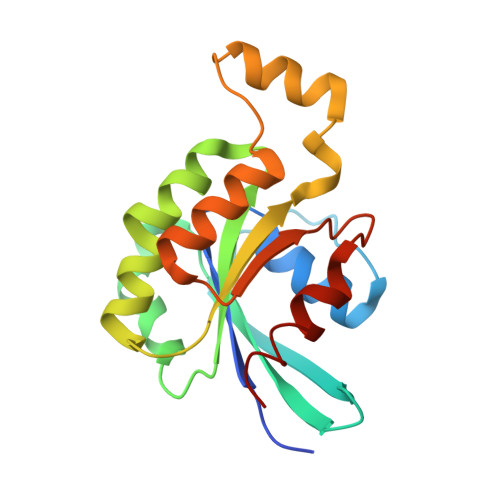
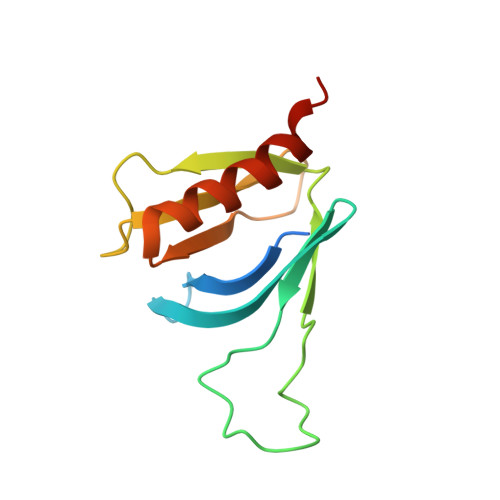
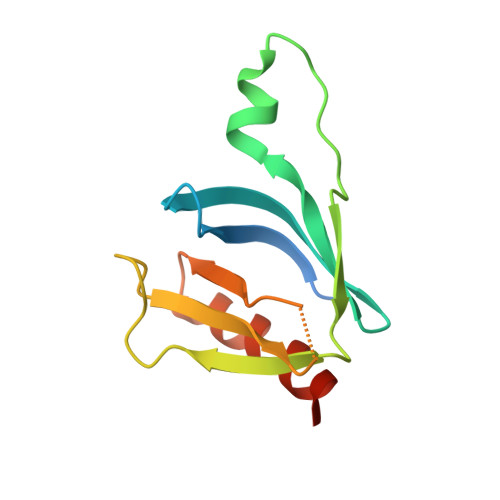
Structural Insights Into Formation of an Active Signaling Complex between Rac and Phospholipase C Gamma 2.
Bunney, T.D., Opaleye, O., Roe, S.M., Vatter, P., Baxendale, R.W., Walliser, C., Everett, K.L., Josephs, M.B., Christow, C., Rodrigues-Lima, F., Gierschik, P., Pearl, L.H., Katan, M.(2009) Mol Cell 34: 223
- PubMed: 19394299
- DOI: https://doi.org/10.1016/j.molcel.2009.02.023
- Primary Citation of Related Structures:
2W2T, 2W2V, 2W2W, 2W2X - PubMed Abstract:
Rho family GTPases are important cellular switches and control a number of physiological functions. Understanding the molecular basis of interaction of these GTPases with their effectors is crucial in understanding their functions in the cell. Here we present the crystal structure of the complex of Rac2 bound to the split pleckstrin homology (spPH) domain of phospholipase C-gamma(2) (PLCgamma(2)). Based on this structure, we illustrate distinct requirements for PLCgamma(2) activation by Rac and EGF and generate Rac effector mutants that specifically block activation of PLCgamma(2), but not the related PLCbeta(2) isoform. Furthermore, in addition to the complex, we report the crystal structures of free spPH and Rac2 bound to GDP and GTPgammaS. These structures illustrate a mechanism of conformational switches that accompany formation of signaling active complexes and highlight the role of effector binding as a common feature of Rac and Cdc42 interactions with a variety of effectors.
- Section of Cell and Molecular Biology , The Institute of Cancer Research, London, UK. tom.bunney@icr.ac.uk
Organizational Affiliation: