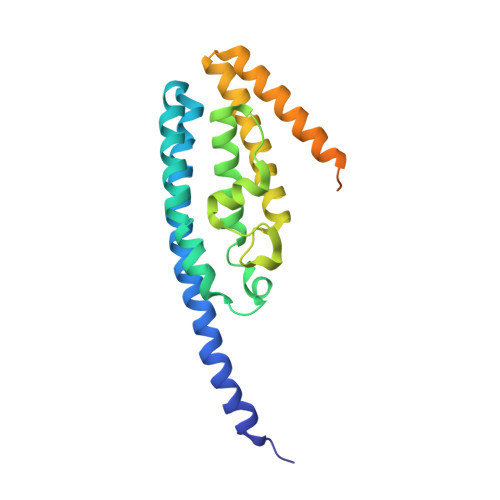
Crystal structures of Drosophila mutant translin and characterization of translin variants reveal the structural plasticity of translin proteins.
Gupta, G.D., Makde, R.D., Rao, B.J., Kumar, V.(2008) FEBS J 275: 4235-4249
- PubMed: 18647346
- DOI: https://doi.org/10.1111/j.1742-4658.2008.06571.x
- Primary Citation of Related Structures:
2QRX, 2QVA - PubMed Abstract:
Translin protein is highly conserved in eukaryotes. Human translin binds both ssDNA and RNA. Its nucleic acid binding site results from a combination of basic regions in the octameric structure. We report here the first biochemical characterization of wild-type Drosophila melanogaster (drosophila) translin and a chimeric translin, and present 3.5 A resolution crystal structures of drosophila P168S mutant translin from two crystal forms. The wild-type drosophila translin most likely exists as an octamer/decamer, and binds to the ssDNA Bcl-CL1 sequence. In contrast, ssDNA binding-incompetent drosophila P168S mutant translin exists as a tetramer. The structures of the mutant translin are identical in both crystal forms, and their C-terminal residues are disordered. The chimeric protein, possessing two nucleic acid binding motifs of drosophila translin, the C-terminal residues of human translin, and serine at position 168, attains the octameric state and binds to ssDNA. The present studies suggest that the oligomeric status of translin critically influences the DNA binding properties of translin proteins.
- High Pressure Physics Division, Bhabha Atomic Research Centre, Mumbai, India.
Organizational Affiliation: