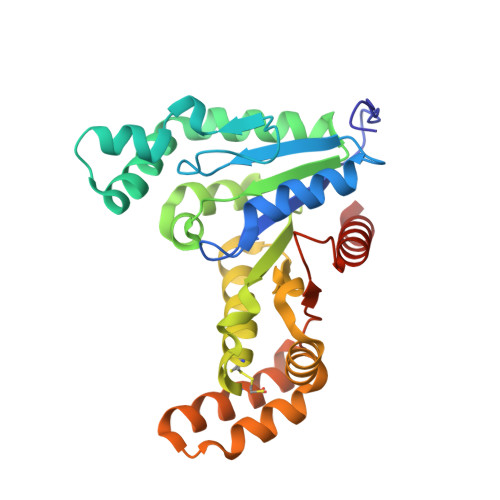
Structural insights into a new homodimeric self-activated GTPase family.
Gras, S., Chaumont, V., Fernandez, B., Carpentier, P., Charrier-Savournin, F., Schmitt, S., Pineau, C., Flament, D., Hecker, A., Forterre, P., Armengaud, J., Housset, D.(2007) EMBO Rep 8: 569-575
- PubMed: 17468740
- DOI: https://doi.org/10.1038/sj.embor.7400958
- Primary Citation of Related Structures:
1YR6, 1YR7, 1YR8, 1YR9, 1YRA, 1YRB, 2OXR - PubMed Abstract:
The human XAB1/MBDin GTPase and its close homologues form one of the ten phylogenetically distinct families of the SIMIBI (after signal recognition particle, MinD and BioD) class of phosphate-binding loop NTPases. The genomic context and the partners identified for the archaeal and eukaryotic homologues indicate that they are involved in genome maintenance--DNA repair or replication. The crystal structure of PAB0955 from Pyrococcus abyssi shows that, unlike other SIMIBI class G proteins, these highly conserved GTPases are homodimeric, regardless of the presence of nucleotides. The nucleotide-binding site of PAB0955 is rather rigid and its conformation is closest to that of the activated SRP G domain. One insertion to the G domain bears a strictly conserved GPN motif, which is part of the catalytic site of the other monomer and stabilizes the phosphate ion formed. Owing to this unique functional feature, we propose to call this family as GPN-loop GTPase.
- Laboratoire de Cristallographie et Cristallogénèse des Protéines, Institut de Biologie Structurale J.-P. Ebel, CEA-CNRS-UJF, UMR 5075, 41, Rue Jules Horowitz, F-38027 Grenoble, France.
Organizational Affiliation: