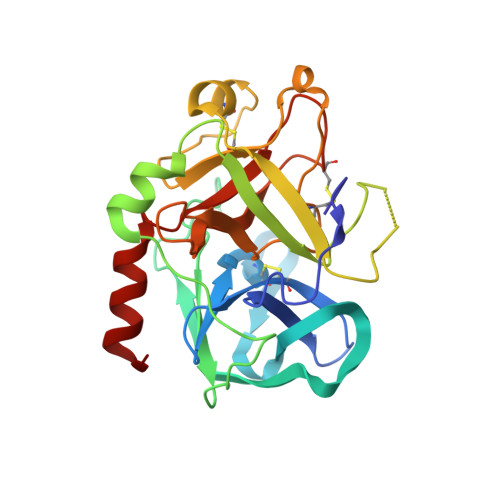
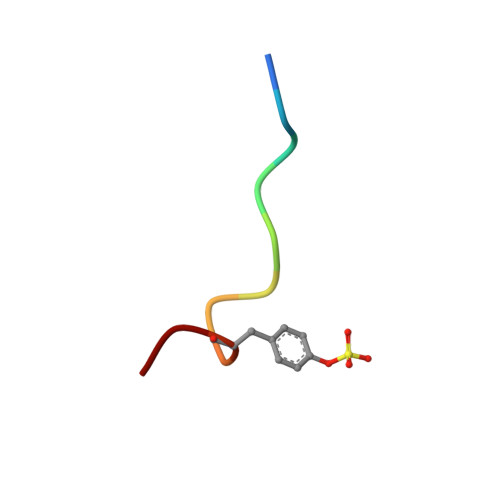
Design of potent selective zinc-mediated serine protease inhibitors.
Katz, B.A., Clark, J.M., Finer-Moore, J.S., Jenkins, T.E., Johnson, C.R., Ross, M.J., Luong, C., Moore, W.R., Stroud, R.M.(1998) Nature 391: 608-612
- PubMed: 9468142
- DOI: https://doi.org/10.1038/35422
- Primary Citation of Related Structures:
1C1N, 1C1O, 1C1P, 1C1Q, 1C1R, 1C1T, 1C1U, 1C1V, 1C1W, 1C2D, 1C2E, 1C2F, 1C2G, 1C2H, 1C2I, 1C2J, 1C2K, 1C2L, 1C2M, 1XUF, 1XUG, 1XUH, 1XUI, 1XUJ, 1XUK - PubMed Abstract:
Many serine proteases are targets for therapeutic intervention because they often play key roles in disease. Small molecule inhibitors of serine proteases with high affinity are especially interesting as they could be used as scaffolds from which to develop drugs selective for protease targets. One such inhibitor is bis(5-amidino-2-benzimidazolyl)methane (BABIM), standing out as the best inhibitor of trypsin (by a factor of over 100) in a series of over 60 relatively closely related analogues. By probing the structural basis of inhibition, we discovered, using crystallographic methods, a new mode of high-affinity binding in which a Zn2+ ion is tetrahedrally coordinated between two chelating nitrogens of BABIM and two active site residues, His57 and Ser 195. Zn2+, at subphysiological levels, enhances inhibition by over 10(3)-fold. The distinct Zn2+ coordination geometry implies a strong dependence of affinity on substituents. This unique structural paradigm has enabled development of potent, highly selective, Zn2+-dependent inhibitors of several therapeutically important serine proteases, using a physiologically ubiquitous metal ion.
- Arris Pharmaceutical Corporation, South San Francisco, California 94080, USA. bak@arris.com
Organizational Affiliation: