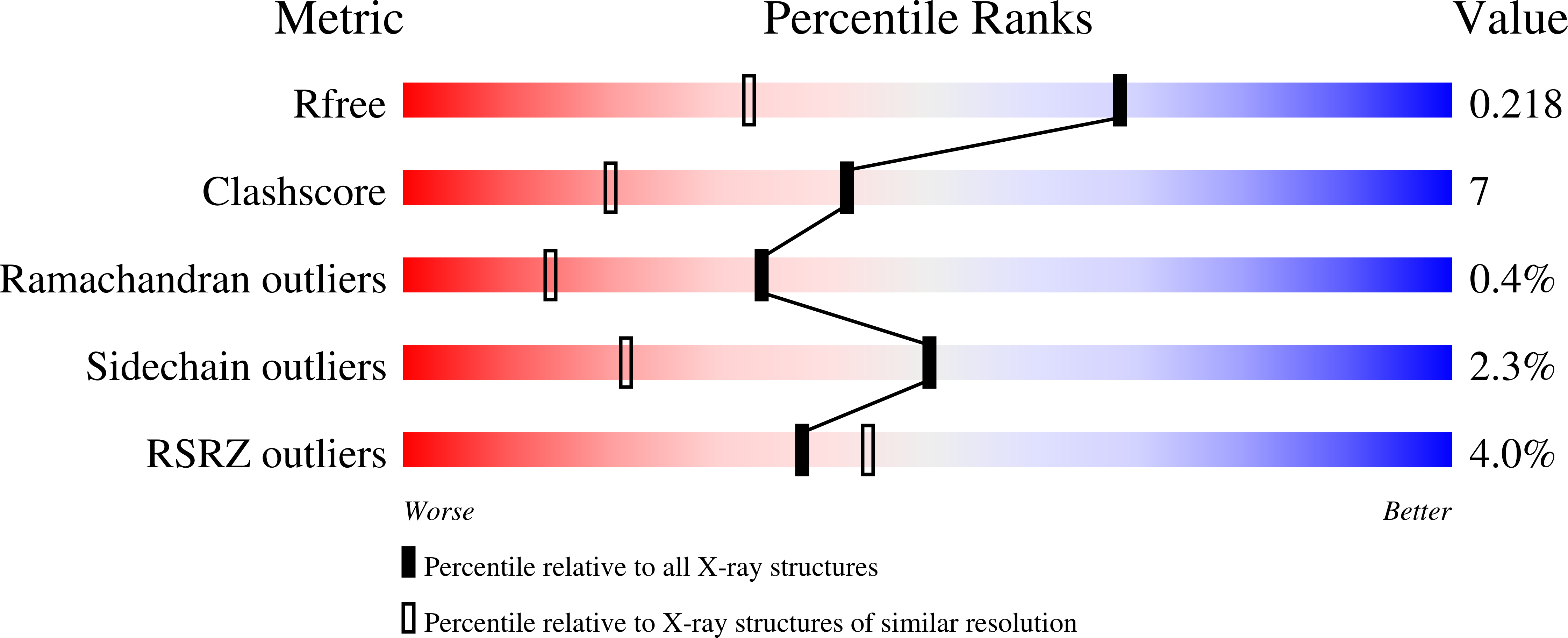
Iterative approach to computational enzyme design.
Privett, H.K., Kiss, G., Lee, T.M., Blomberg, R., Chica, R.A., Thomas, L.M., Hilvert, D., Houk, K.N., Mayo, S.L.(2012) Proc Natl Acad Sci U S A 109: 3790-3795
- PubMed: 22357762
- DOI: https://doi.org/10.1073/pnas.1118082108
- Primary Citation of Related Structures:
3NYD, 3NYZ, 3NZ1, 3O2L - PubMed Abstract:
A general approach for the computational design of enzymes to catalyze arbitrary reactions is a goal at the forefront of the field of protein design. Recently, computationally designed enzymes have been produced for three chemical reactions through the synthesis and screening of a large number of variants. Here, we present an iterative approach that has led to the development of the most catalytically efficient computationally designed enzyme for the Kemp elimination to date. Previously established computational techniques were used to generate an initial design, HG-1, which was catalytically inactive. Analysis of HG-1 with molecular dynamics simulations (MD) and X-ray crystallography indicated that the inactivity might be due to bound waters and high flexibility of residues within the active site. This analysis guided changes to our design procedure, moved the design deeper into the interior of the protein, and resulted in an active Kemp eliminase, HG-2. The cocrystal structure of this enzyme with a transition state analog (TSA) revealed that the TSA was bound in the active site, interacted with the intended catalytic base in a catalytically relevant manner, but was flipped relative to the design model. MD analysis of HG-2 led to an additional point mutation, HG-3, that produced a further threefold improvement in activity. This iterative approach to computational enzyme design, including detailed MD and structural analysis of both active and inactive designs, promises a more complete understanding of the underlying principles of enzymatic catalysis and furthers progress toward reliably producing active enzymes.
Organizational Affiliation:
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125, USA.