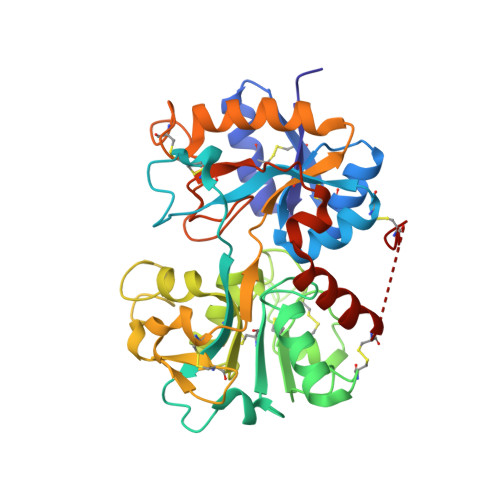
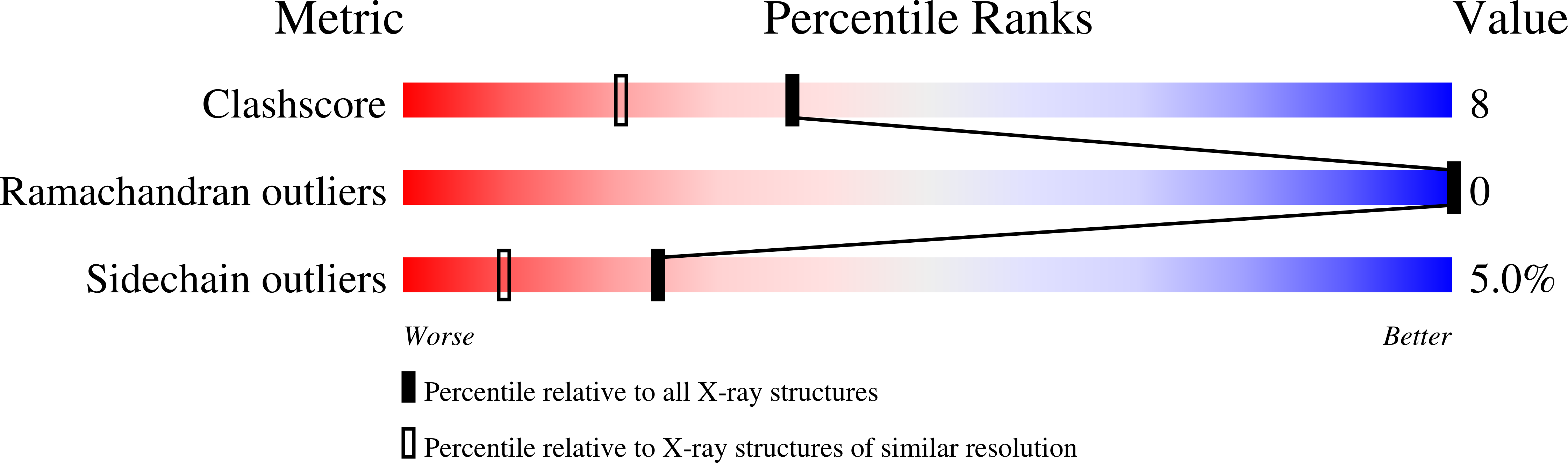Crystal structure of the C-terminal lobe of bovine lactoferrin complexed with O-alpha-D-Glucopyranosyl-(1 3)-alpha-D-fructofuranosyl-(2 1)-alpha-D-glucopyranoside at 1.93 A resolution
Mir, R., Singh, N., Sinha, M., Sharma, S., Kaur, P., Singh, T.P.To be published.