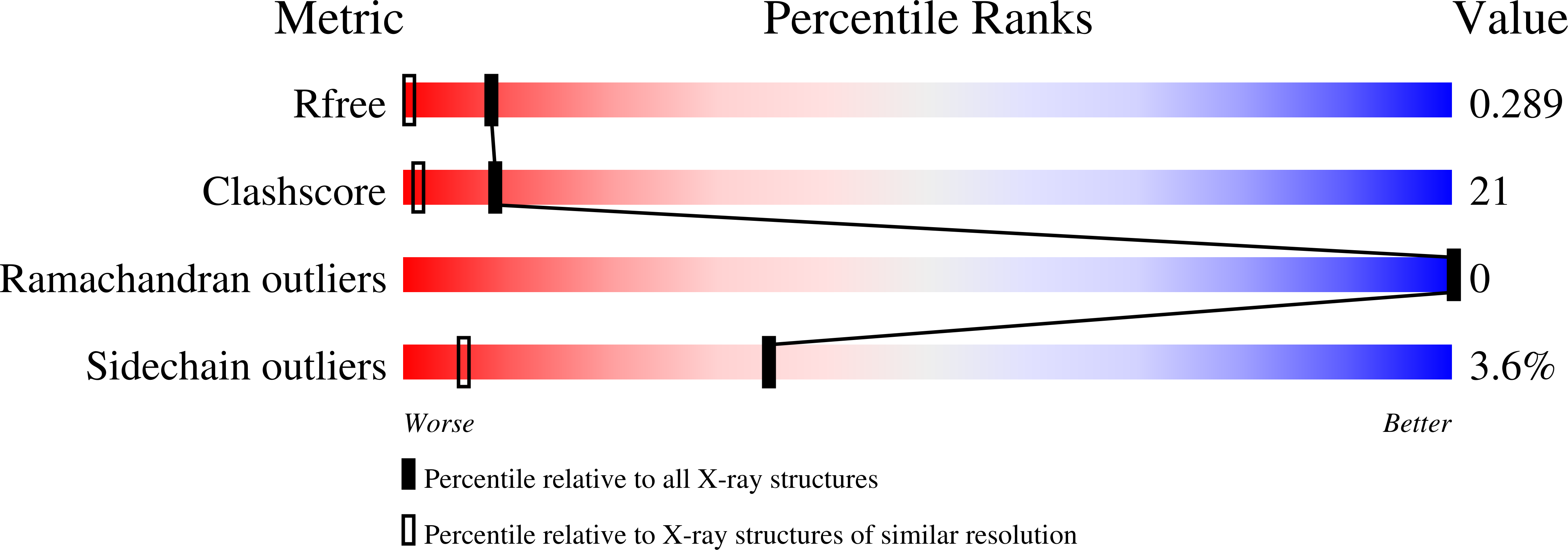
Structural analysis of the carboxy terminal PH domain of pleckstrin bound to D-myo-inositol 1,2,3,5,6-pentakisphosphate.
Jackson, S.G., Zhang, Y., Haslam, R.J., Junop, M.S.(2007) BMC Struct Biol 7: 80-80
- PubMed: 18034889
- DOI: https://doi.org/10.1186/1472-6807-7-80
- Primary Citation of Related Structures:
2I5C, 2I5F - PubMed Abstract:
Pleckstrin homology (PH) domains are one of the most prevalent domains in the human proteome and represent the major phosphoinositide-binding module. These domains are often found in signaling proteins and function predominately by targeting their host proteins to the cell membrane. Inositol phosphates, which are structurally similar to phosphoinositides, are not only known to play a role as signaling molecules but are also capable of being bound by PH domains. In the work presented here it is shown that the addition of commercial myo-inositol hexakisphosphate (IP6) inhibited the binding of the carboxy terminal PH domain of pleckstrin (C-PH) to phosphatidylinositol 3,4-bisphosphate with an IC50 of 7.5 muM. In an attempt to characterize this binding structurally, C-PH was crystallized in the presence of IP6 and the structure was determined to 1.35 A. Examination of the resulting electron density unexpectedly revealed the bound ligand to be D-myo-inositol 1,2,3,5,6-pentakisphosphate. The discovery of D-myo-inositol 1,2,3,5,6-pentakisphosphate in the crystal structure suggests that the inhibitory effects observed in the binding studies may be due to this ligand rather than IP6. Analysis of the protein-ligand interaction demonstrated that this myo-inositol pentakisphosphate isomer interacts specifically with protein residues known to be involved in phosphoinositide binding. In addition to this, a structural alignment of other PH domains bound to inositol phosphates containing either four or five phosphate groups revealed that the majority of phosphate groups occupy conserved locations in the binding pockets of PH domains. These findings, taken together with other recently reported studies suggest that myo-inositol pentakisphosphates could act to regulate PH domain-phosphoinositide interactions by directly competing for binding, thus playing an important role as signaling molecules.
Organizational Affiliation:
Department of Biochemistry and Biomedical Sciences, McMaster University, Hamilton, Canada. jacksosg@mcmaster.ca