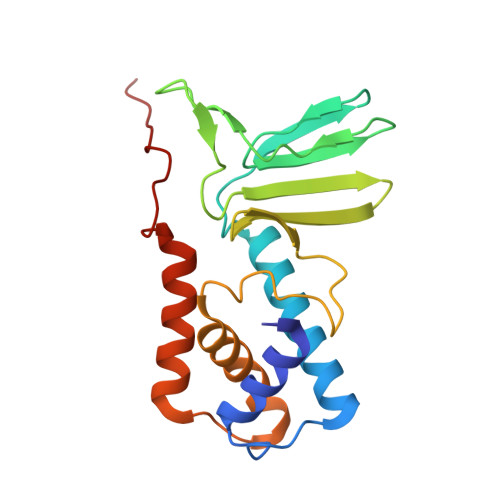
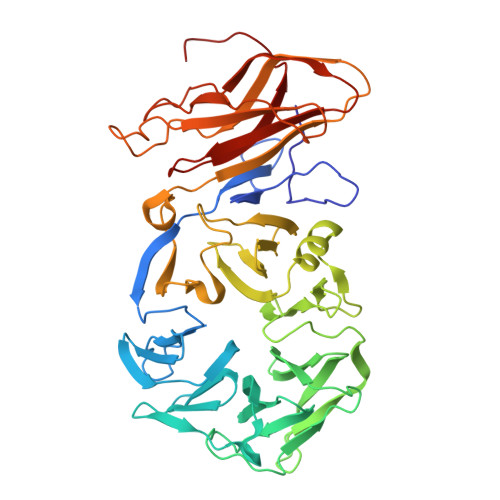
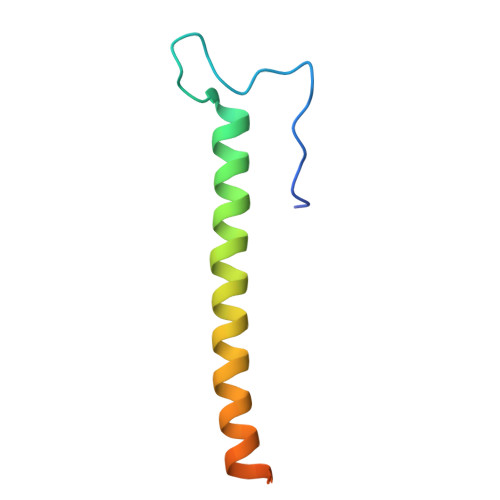
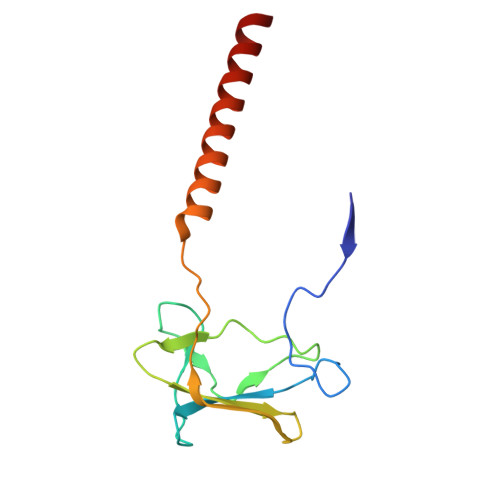
Cryo-EM structure of cyanopodophage Pan3 reveals a modular tail architecture for host recognition.
Hou, P., Zhu, J., Yu, R.C., Yang, F., Du, K., Li, J., Kong, W.W., Wang, J., Chen, Y., Zhou, C.Z., Jiang, Y.L.(2025) Structure
- PubMed: 41380677
- DOI: https://doi.org/10.1016/j.str.2025.11.012
- Primary Citation of Related Structures:
9VYE, 9VYF, 9VYG, 9VYH, 9VZI - PubMed Abstract:
Cyanophages, which are bacteriophages that specifically infect host cyanobacteria, also utilize the tail to initiate host recognition and adsorption. Owing to the limited structural information on cyanophages, our understanding of the mechanism by which cyanophages specifically recognize their hosts remains largely unknown. Here, we determined the intact cryoelectron microscopy structure of a freshwater cyanopodophage Pan3, which consists of an icosahedral shell and a short tail comprising four modular components: the dodecameric adaptor, hexameric nozzle, trimeric needle, and six heterohexameric tailspikes. Notably, each tailspike features an SGNH esterase domain fused to a lectin domain, forming a continuous groove complementary to the host lipopolysaccharide. These findings provide insights into the receptor engagement in Podoviridae, and establish a structural framework for cyanophage and host interactions that may guide future antibacterial interventions against harmful blooms.
- Hefei National Research Center for Physical Sciences at the Microscale, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China.
Organizational Affiliation: