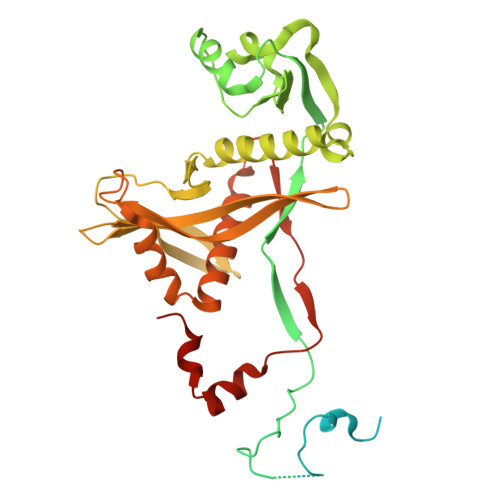
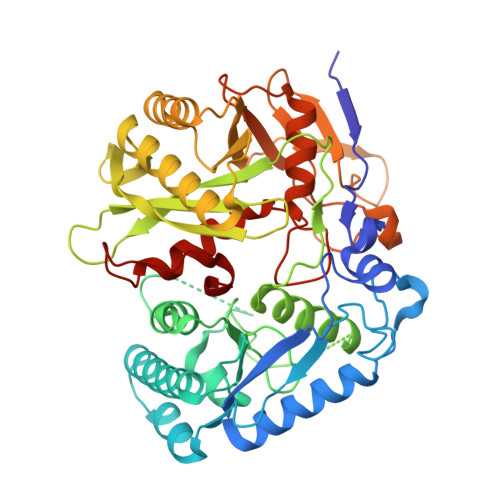
Activation of the SPARDA defense system by filament assembly using a beta-relay signaling mechanism widespread in prokaryotic Argonautes.
Jurgelaitis, E., Zagorskaite, E., Kopustas, A., Asmontas, S., Manakova, E., Dalgediene, I., Tylenyte, U., Silanskas, A., Toliusis, P., Grybauskas, A., Tutkus, M., Venclovas, C., Zaremba, M.(2025) Cell Res 35: 1056-1078
- PubMed: 41298897
- DOI: https://doi.org/10.1038/s41422-025-01198-1
- Primary Citation of Related Structures:
9QBL, 9QBP, 9QBQ, 9QCC - PubMed Abstract:
Present in all three domains of life, Argonaute proteins use short oligonucleotides as guides to recognize complementary nucleic acid targets. In eukaryotes, Argonautes are involved in RNA silencing, whereas in prokaryotes, they function in host defense against invading DNA. Here, we show that SPARDA (short prokaryotic Argonaute, DNase associated) systems from Xanthobacter autotrophicus (Xau) and Enhydrobacter aerosaccus (Eae) function in anti-plasmid defense. Upon activation, SPARDA nonspecifically degrades both invader and genomic DNA, causing host death, thereby preventing further spread of the invader in the population. X-ray structures of the apo Xau and EaeSPARDA complexes show that they are dimers, unlike other apo short pAgo systems, which are monomers. We show that dimerization in the apo state is essential for inhibition of XauSPARDA activity. We demonstrate by cryo-EM that activated XauSPARDA forms a filament. Upon activation, the recognition signal of the bound guide/target duplex is relayed to other functional XauSPARDA sites through a structural region that we termed the "beta-relay". Owing to dramatic conformational changes associated with guide/target binding, XauSPARDA undergoes a "dimer-monomer-filament" transition as the apo dimer dissociates into the guide/target-loaded monomers that subsequently assemble into the filament. Within the activated filament, the DREN nuclease domains form tetramers that are poised to cleave dsDNA. We show that other SPARDAs also form filaments during activation. Furthermore, we identify the presence of the beta-relay in pAgo from all clades, providing new insights into the structural mechanisms of pAgo proteins. Taken together, these findings reveal the detailed structural mechanism of SPARDA and highlight the importance of the beta-relay mechanism in signal transduction in Argonautes.
- Institute of Biotechnology, Life Sciences Center, Vilnius University, Vilnius, Lithuania.
Organizational Affiliation: