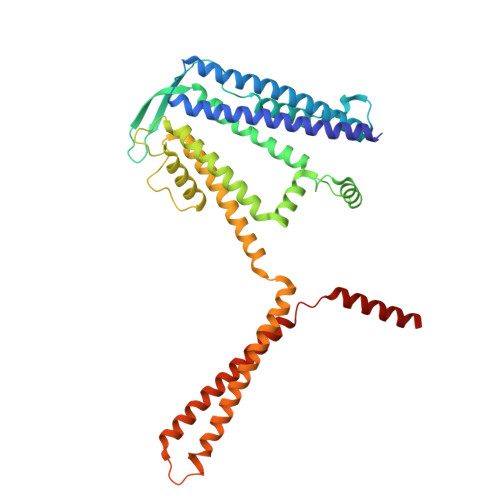
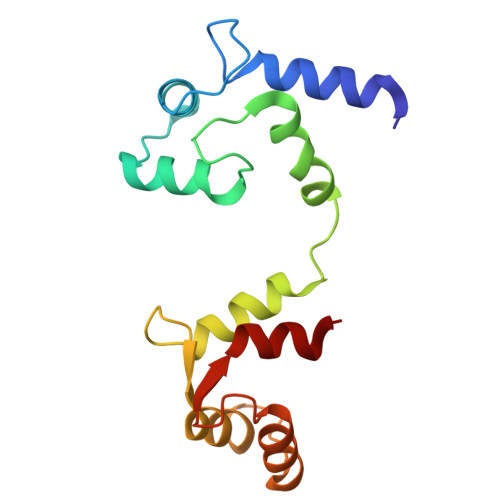
Structural basis for the subtype-selectivity of K Ca 2.2 channel activators.
Nam, Y.W., Ramanishka, A., Xu, Y., Yasuda, R.M.H., Nasburg, J.A., Im, D., Cui, M., Chandy, K.G., Wulff, H., Zhang, M.(2026) Nat Commun 17: 531-531
- PubMed: 41507196
- DOI: https://doi.org/10.1038/s41467-025-67232-3
- Primary Citation of Related Structures:
9O7S, 9O85, 9O93, 9OA8, 9Y5Q, 9YDZ - PubMed Abstract:
Small-conductance (K Ca 2.2) and intermediate-conductance (K Ca 3.1) Ca 2+ -activated K + channels are gated by a Ca 2+ -calmodulin dependent mechanism. NS309 potentiates the activity of both K Ca 2.2 and K Ca 3.1, while rimtuzalcap selectively activates K Ca 2.2. Rimtuzalcap has been used in clinical trials for the treatment of spinocerebellar ataxia and essential tremor. We report cryo-electron microscopy structures of NS309-bound K Ca 2.2 and K Ca 3.1, in addition to structures of rimtuzalcap-bound K Ca 2.2 and mutant K Ca 3.1_R355K. The different conformations of calmodulin and the cytoplasmic HC helices in the two channels underlie the subtype-selectivity of rimtuzalcap for K Ca 2.2. NS309 binds to pre-existing pockets in both channels, while the bulkier rimtuzalcap binds in an induced-fit pocket in K Ca 2.2 requiring conformational changes. In K Ca 2.2, calmodulin's N-lobes are sufficiently far apart to enable conformational changes to accommodate either NS309 or rimtuzalcap. In K Ca 3.1, calmodulin's N-lobes are closer to each other and constrained by K Ca 3.1's HC helices, which allows binding of NS309 but not rimtuzalcap. Replacement of arginine-355 in K Ca 3.1's HB helix with lysine (K Ca 3.1_R355K) allows the binding of rimtuzalcap and renders the mutant channel sensitive to rimtuzalcap. These structures provide a framework for structure-based drug design targeting K Ca 2.2 channels.
- Department of Biomedical and Pharmaceutical Sciences, Chapman University School of Pharmacy, Irvine, CA, USA.
Organizational Affiliation: