SNARE disassembly requires Sec18/NSF side loading.
Khan, Y.A., White, K.I., Pfuetzner, R.A., Singal, B., Esquivies, L., Mckenzie, G., Liu, F., DeLong, K., Choi, U.B., Montabana, E., Mclaughlin, T., Wickner, W.T., Brunger, A.T.(2025) Nat Struct Mol Biol 32: 1708-1720
- PubMed: 40604310
- DOI: https://doi.org/10.1038/s41594-025-01590-w
- Primary Citation of Related Structures:
9CRU, 9CRX, 9N22, 9NG2, 9NLU, 9NLW, 9NLY, 9NLZ, 9NM1, 9NUD, 9NUE, 9NUZ, 9NV0, 9NV1, 9NV9, 9NVD - PubMed Abstract:
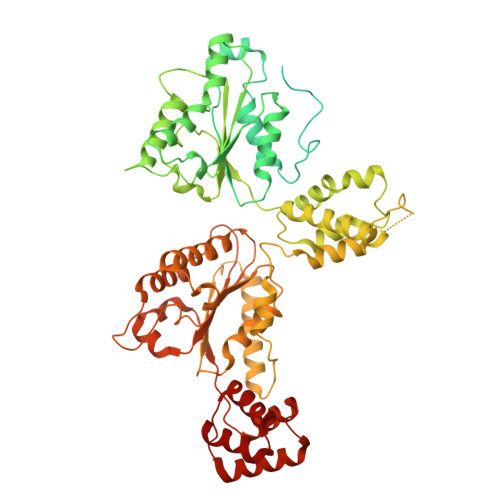
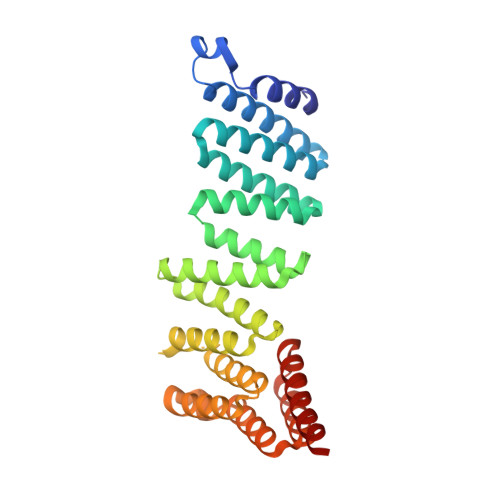
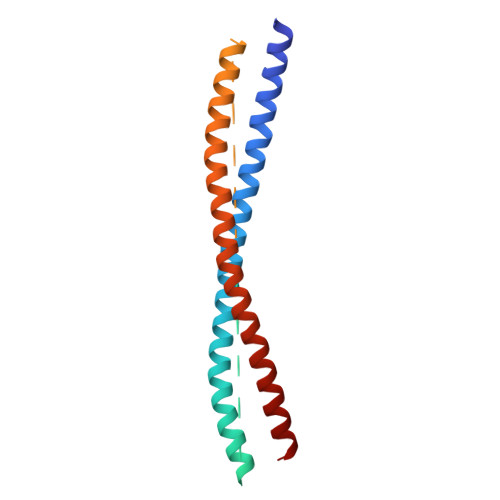
SNARE (soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor) proteins drive membrane fusion at different cell compartments as their core domains zipper into a parallel four-helix bundle. After fusion, these bundles are disassembled by the AAA+ (ATPase associated with diverse cellular activities) protein Sec18/NSF and its adaptor Sec17/α-SNAP to make them available for subsequent rounds of membrane fusion. SNARE domains are often flanked by C-terminal transmembrane or N-terminal domains. Previous structures of the NSF-α-SNAP-SNARE complex revealed binding to the D1 ATPase pore, posing a topological constraint as SNARE transmembrane domains would prevent complete substrate threading as suggested for other AAA+ systems. Using mass spectrometry in yeast cells, we show N-terminal SNARE domain interactions with Sec18, exacerbating this topological issue. We present cryo-electron microscopy (cryo-EM) structures of a yeast SNARE complex, Sec18 and Sec17 in a nonhydrolyzing condition, which show SNARE Sso1 threaded through the D1 and D2 ATPase rings of Sec18, with its folded, N-terminal Habc domain interacting with the D2 ring. This domain does not unfold during Sec18/NSF activity. Cryo-EM structures under hydrolyzing conditions revealed substrate-released and substrate-free states of Sec18 with a coordinated opening in the side of the ATPase rings. Thus, Sec18/NSF operates by substrate side loading and unloading topologically constrained SNARE substrates.
- Department of Molecular and Cellular Physiology, Stanford University, Stanford, CA, USA. yousuf@stanford.edu.
Organizational Affiliation: