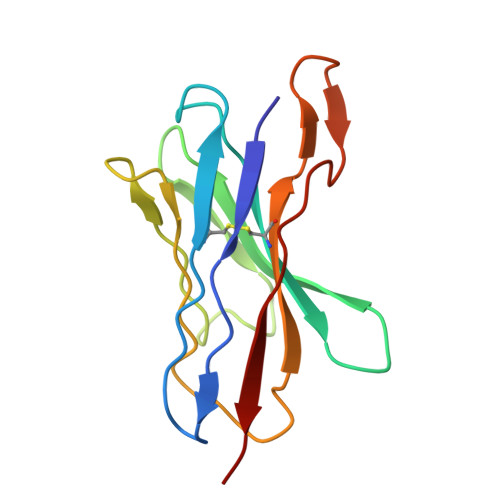
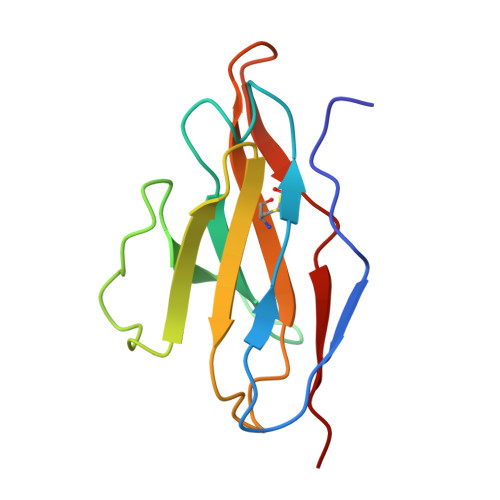
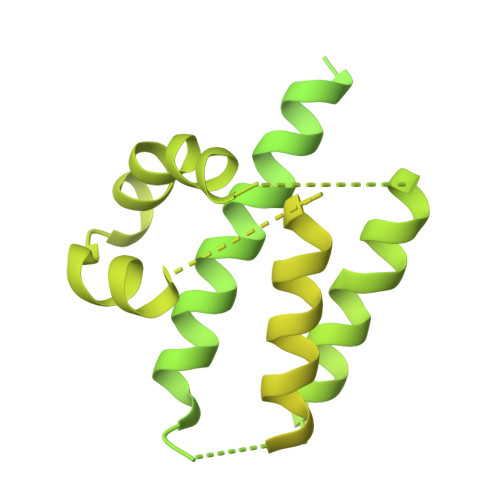
Identification of broadly inhibitory anti-PfEMP1 antibodies by mass spectrometry sequencing of plasma IgG from a malaria-exposed child.
Turner, L., Nunez de Villavicencio Diaz, T., Raghavan, S.S.R., Kana, I.H., Lyimo, E., Reitzel, C., Wang, C.W., Berube, E., Jensen, R.W., Loeffler, J.R., Fernandez-Quintero, M.L., Theander, T.G., Lusingu, J.P.A., Le Bihan, T., Han, X., Minja, D.T.R., Ward, A.B., Ma, B., Lavstsen, T.(2025) Proc Natl Acad Sci U S A 122: e2508744122-e2508744122
- PubMed: 40833410
- DOI: https://doi.org/10.1073/pnas.2508744122
- Primary Citation of Related Structures:
9NAQ - PubMed Abstract:
Plasmodium falciparum pathology is driven by the accumulation of parasite-infected erythrocytes in blood capillaries. This sequestration process is mediated by the parasite's P. falciparum erythrocyte membrane protein 1 (PfEMP1) adhesins, which bind select endothelial cell receptors. A subset of PfEMP1 binding human endothelial protein C receptor (EPCR) through their cysteine-rich interdomain region alpha 1 (CIDRα1) domains drives the pathogenesis to severe malaria. Despite high sequence diversity among CIDRα1 domains, individuals living in malaria-endemic regions become immune to severe disease in part through acquisition of antibodies inhibiting the PfEMP1-EPCR interaction. Here, we demonstrate an approach to identify pathogen-specific human monoclonal antibodies from plasma, combining mass spectrometry analysis of antigen-purified polyclonal plasma IgG and Ig transcript sequencing. We identified a clonal family of broadly reactive and EPCR binding-inhibitory human monoclonal antibodies against CIDRα1. The antibodies, isolated from a 9-y-old child, exhibited potent inhibition of EPCR binding broadly across CIDRα1 domains as well as binding of infected erythrocytes to EPCR. Structural analysis of one antibody variant complexed with CIDRα1 revealed a shared epitope of the clonal antibody family overlapping the EPCR binding site and the epitopes of two previously identified monoclonal antibodies, C7 and C74, with similar functional patterns. However, although C7, C74, and 110-3 antibodies all depend on the same few residues conserved in CIDRα1 to retain EPCR binding, the 110-3 antibodies contact additional variable residues, reducing their breadth of reactivity across the CIDRα1 family. These data bolster the hypothesis that broadly inhibitory antibodies against severe malaria-associated PfEMP1 target similar epitopes and are commonly developed in malaria-exposed individuals.
- Centre for Translational Medicine and Parasitology, Department of Immunology and Microbiology, University of Copenhagen, Copenhagen 2200, Denmark.
Organizational Affiliation: