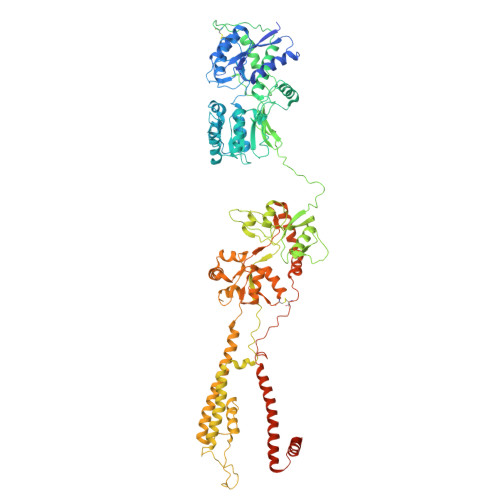
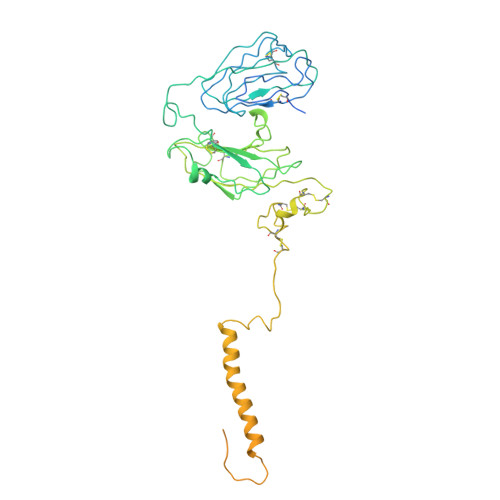
Activation of kainate receptor GluK2-Neto2 complex.
Gangwar, S.P., Yelshanskaya, M.V., Yen, L.Y., Newton, T.P., Sobolevsky, A.I.(2025) Nat Struct Mol Biol 32: 2176-2184
- PubMed: 40846810
- DOI: https://doi.org/10.1038/s41594-025-01656-9
- Primary Citation of Related Structures:
9N4L, 9N4M, 9N4N, 9N4O, 9N4P, 9N4Q, 9N4R, 9N4S, 9N4T - PubMed Abstract:
Kainate receptors (KARs) are tetrameric, ligand-gated ion channels of the ionotropic glutamate receptor family that mediate excitatory neurotransmission and modulate neuronal circuits and synaptic plasticity during development of the central nervous system. KARs are implicated in psychiatric and neurological diseases and represent a target of therapeutic intervention. Native KARs form complexes with neuropilin and tolloid-like auxiliary subunits (Neto1 and Neto2), which modulate their function, trafficking and synaptic localization. Here we present structures of rat GluK2 KAR in the apo closed state and in the open states activated by agonist kainate and positive allosteric modulator BPAM344, solved in the presence and absence of Neto2 using time-resolved cryo-electron microscopy. While the binding of Neto2 does not change the behavior of individual or dimeric ligand-binding domains (LBDs) or the ion channel, it prevents tightening of the interface between two LBD dimers during activation and slows the kinetics of deactivation. Our structures illuminate the mechanism of KAR activation and its modulation by Neto2.
- Department of Biochemistry and Molecular Biophysics, Columbia University, New York, NY, USA.
Organizational Affiliation: