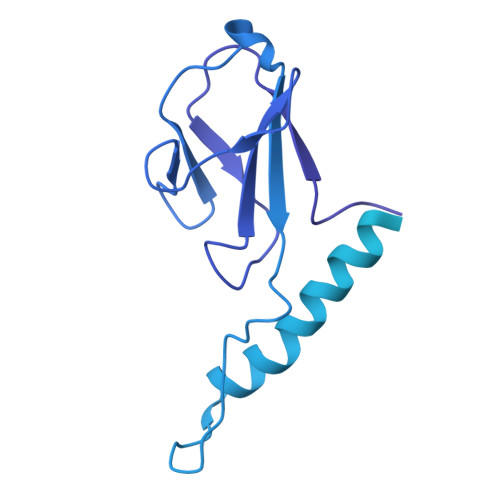
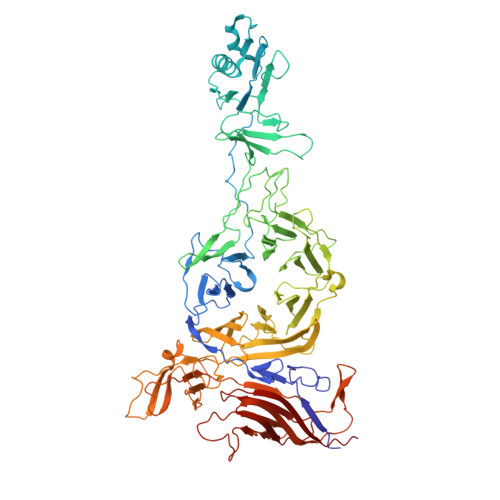
Conformational changes in and translocation of small proteins: insights into the ejection mechanism of podophages.
Zheng, J., Xiao, H., Pang, H., Wang, L., Song, J., Chen, W., Cheng, L., Liu, H.(2025) J Virol 99: e0124924-e0124924
- PubMed: 39704524
- DOI: https://doi.org/10.1128/jvi.01249-24
- Primary Citation of Related Structures:
9JYY, 9JYZ, 9JZ0 - PubMed Abstract:
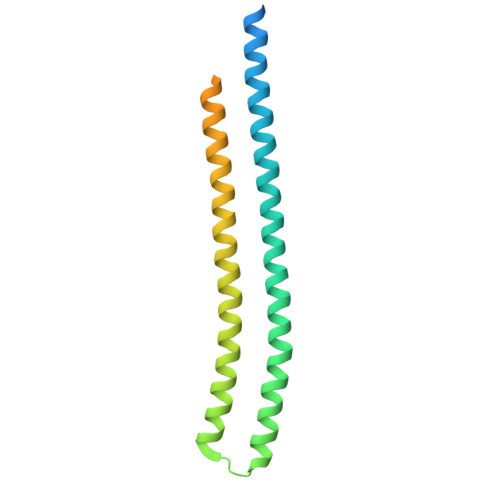
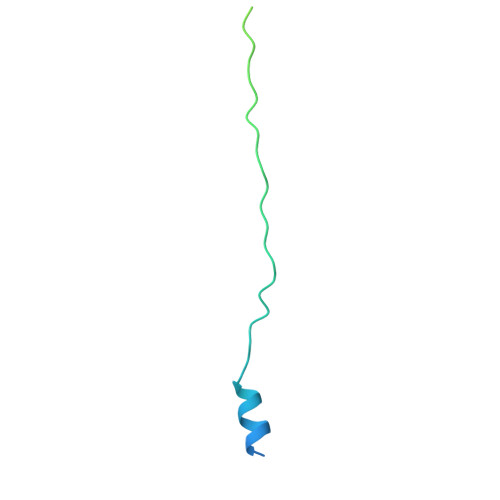
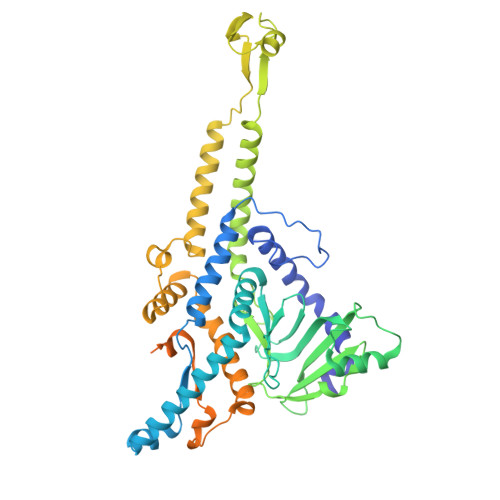
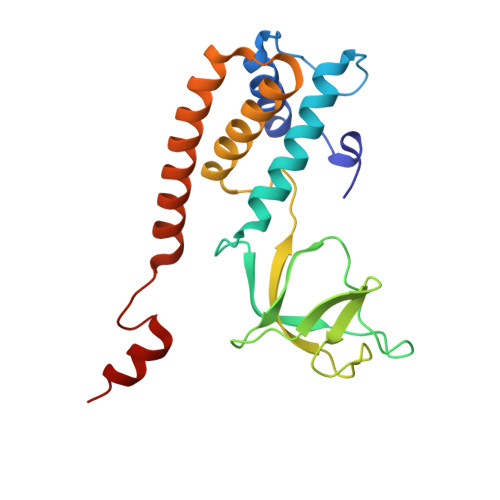
Podophage tails are too short to span the cell envelope during infection. Consequently, podophages initially eject the core proteins within the head for the formation of an elongated trans-envelope channel for DNA ejection. Although the core proteins of bacteriophage T7 have been resolved at near-atomic resolution, the mechanisms of core proteins and DNA ejection remain to be fully elucidated. In this study, we provided improved structures of core proteins in mature T7 and the portal-tail complex in lipopolysaccharide-induced DNA-ejected T7 to resolutions of approximately 3 Å. Using these structures, we identified three small proteins, namely gp14, gp6.7, and gp7.3, and illustrated the conformational changes in and translocation of these proteins from the mature to DNA-ejected states. Our structures indicate that gp6.7, which participates in the assembly of the core and trans-envelope channel, is a core protein, and that gp7.3 serves as a structural scaffold to assist the assembly of the nozzle into the adaptor. Podophage T7 core proteins form an elongated trans-envelope channel for genomic DNA delivery into the host cell. The structures of the core proteins within the mature T7 and assembled in the periplasmic tunnel form in the DNA-ejected T7 have been resolved previously. Here, we resolved the structures of two new structural proteins (gp6.7 and gp7.3) within mature T7 and receptor-induced DNA-ejected T7. The gp6.7 protein participates in the assembly of the core complex within mature T7 and the trans-envelope channel during T7 infection; therefore, gp6.7 is a core protein. Before T7 infection, gp7.3 plays a role in promoting the assembly of the nozzle into the adaptor.
- Institute of Interdisciplinary Studies, Key Laboratory for Matter Microstructure and Function of Hunan Province, Key Laboratory of Low-dimensional Quantum Structures and Quantum Control, School of Physics and Electronics, Hunan Normal University, Changsha, China.
Organizational Affiliation: