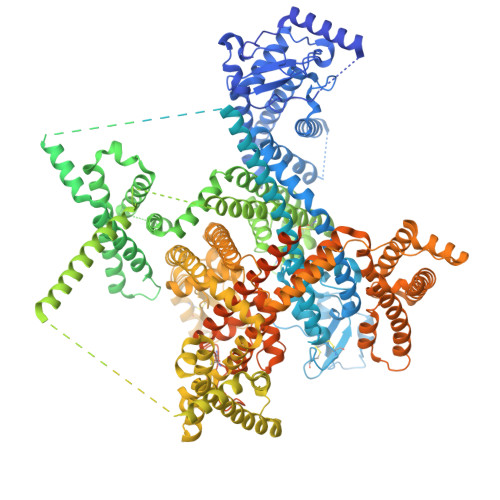
Critical role of extracellular loops in differential modulations of TTX-sensitive and TTX-resistant Na v channels.
Wu, T., Yang, X., Jin, X., Yan, N., Li, Z.(2025) Proc Natl Acad Sci U S A 122: e2510355122-e2510355122
- PubMed: 40768348
- DOI: https://doi.org/10.1073/pnas.2510355122
- Primary Citation of Related Structures:
9ITH, 9ITI - PubMed Abstract:
The cardiac voltage-gated sodium channel Na v 1.5 is resistant to tetrodotoxin (TTXr). Here, we report a cryo-electron microscopy (cryo-EM) structure of wild-type human Na v 1.5, coexpressed with the β1 auxiliary subunit and treated with high-concentration TTX, at 3.4 Å resolution. Structural comparison reveals the molecular determinants for the distinct responses to TTX as well as β subunits between TTXr and TTX-sensitive (TTXs) Na v channels. A conserved cation-π interaction between the guanidinium group of TTX and Tyr or Phe on the P2 I helix in TTXs Na v channels is lost in all TTXr subtypes owing to the replacement by Cys/Ser at the corresponding locus, explaining their differential TTX sensitivities. The β1 subunit is invisible in the EM map. Comparison of Na v 1.5 with Na v 1.7 and Na v 1.8, which are, respectively, TTXs and TTXr, identifies four sites on the extracellular loops (ECLs) that may account for their different β1-binding abilities. When the corresponding residues in TTXs Na v 1.7 are replaced with those from Na v 1.5, the modulatory effects of β1 on channel activation and inactivation are diminished. Consistently, β1 is absent in the 3D EM reconstruction of this Na v 1.7 mutant. Together with our previous structure-guided discovery that TTXr channels lack a Cys on the ECL II for disulfide bond formation with β2 or β4, the structure-function relationship studies underscore the importance of the ECLs in the mechanistic distinctions between TTXs and TTXr Na v channels. The ECLs may be further explored for the development of subtype-specific drugs.
- State Key Laboratory of Membrane Biology, Beijing Advanced Innovation Center for Structural Biology, Tsinghua-Peking Joint Center for Life Sciences, School of Life Sciences, Tsinghua University, Beijing 100084, China.
Organizational Affiliation: